
Which of the following substances is not of $3 - {\text{ethyl 2 - methyl pentane}}$?
(A)
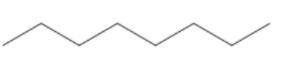
(B)
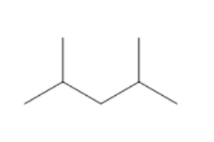
(C)
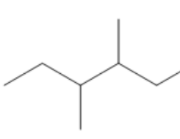
(D) All are isomers



Answer
524.7k+ views
Hint :$3 - {\text{ethyl 2 - methyl pentane}}$ has eight carbon atoms in its structure. Five carbon atoms are present in the parent chain, two carbon atoms in the ethyl group and one carbon atom in the methyl group. First check the number of carbon atoms in all options.
Complete Step By Step Answer:
Count the number of carbon atoms in all the given options. Option A has eight carbon atoms in the parent chain. Option B has only seven carbon atoms in its structure, five carbon atoms are present in the parent chain and one carbon atom in each methyl group. Option C has eight carbon atoms, six carbon atoms are present in the parent chain and one carbon atom in each methyl group. So, we can clearly see that only option B has seven carbon atoms. The compound in option B is not an isomer of $3 - {\text{ethyl 2 - methyl pentane}}$.
Therefore, option B is correct.
Note :
Before solving questions related to isomers, first check the number of carbon atoms. Isomers are molecules with the same molecular formula but have different arrangements in space. The arrangement of atoms varies in different isomers but the number of atoms remains the same. Isomerism is classified into two types. They are constitutional isomers and stereoisomers. Remember that propane has no isomers because it does not have enough carbon atoms to exist in the form of branches. Propane has only one possible structure in nature. All the three bonds present in propane are single bonds and it is an alkane. It is not necessary that isomers should have similar physical and chemical properties.
Complete Step By Step Answer:
Count the number of carbon atoms in all the given options. Option A has eight carbon atoms in the parent chain. Option B has only seven carbon atoms in its structure, five carbon atoms are present in the parent chain and one carbon atom in each methyl group. Option C has eight carbon atoms, six carbon atoms are present in the parent chain and one carbon atom in each methyl group. So, we can clearly see that only option B has seven carbon atoms. The compound in option B is not an isomer of $3 - {\text{ethyl 2 - methyl pentane}}$.
Therefore, option B is correct.
Note :
Before solving questions related to isomers, first check the number of carbon atoms. Isomers are molecules with the same molecular formula but have different arrangements in space. The arrangement of atoms varies in different isomers but the number of atoms remains the same. Isomerism is classified into two types. They are constitutional isomers and stereoisomers. Remember that propane has no isomers because it does not have enough carbon atoms to exist in the form of branches. Propane has only one possible structure in nature. All the three bonds present in propane are single bonds and it is an alkane. It is not necessary that isomers should have similar physical and chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




