
Which of the following structures contains a hemiacetal group?
A.
B.
C.
D.




Answer
579k+ views
Hint: To solve this question, we must first understand and discuss the characteristics of a hemiacetal group. . A hemiacetal group consists of a carbon atom which is attached to a hydroxyl group on one side, and to an ether on the other. Now, we must go through all the options to identify the presence of a hemiacetal in them.
Complete Step-by-Step Answer:
Many people may confuse an acetal group with a hemiacetal group due to the similarities in their names. An acetal group can be identified as the group which has a carbon atom that is attached to two ether groups at the same time. On the other hand, the hemiacetal group is slightly different compared to an acetal group. The general representation of an acetal group can be given as
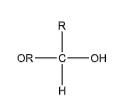
Let us now discuss the options given to us:
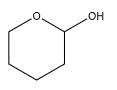
A.
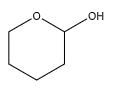
We can observe that one of the carbon atoms has a hydroxyl group at one end. But it has only an oxygen atom on the other end, instead of an ether. Hence, it does not contain a hemiacetal group.
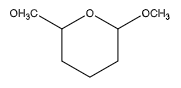
B.
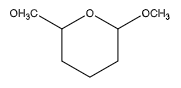
We can observe that two of the carbon atoms have an ether group at one end. But they have only an oxygen atom on the other end, instead of a hydroxyl group. Hence, it does not contain a hemiacetal group.
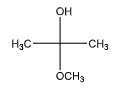
C.
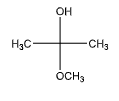
We can observe that one of the carbon atoms is attached to both an ether and a hydroxyl group. Hence, it does contain a hemiacetal group.
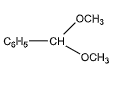
D.
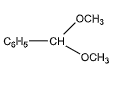
We can observe that one of the carbon atoms has two ether groups at one end. But it has only a benzene molecule on the other end, instead of a hydroxyl group. Hence, it does not contain a hemiacetal group.
Hence, Option C is the correct option
Note: The acetal is derived from a hemiacetal and an alcohol making the second ether group. Hemiacetal Formation: An aldehyde or ketone plus an alcohol may exist in equilibrium with a hemiacetal.
Complete Step-by-Step Answer:
Many people may confuse an acetal group with a hemiacetal group due to the similarities in their names. An acetal group can be identified as the group which has a carbon atom that is attached to two ether groups at the same time. On the other hand, the hemiacetal group is slightly different compared to an acetal group. The general representation of an acetal group can be given as

Let us now discuss the options given to us:
A.

We can observe that one of the carbon atoms has a hydroxyl group at one end. But it has only an oxygen atom on the other end, instead of an ether. Hence, it does not contain a hemiacetal group.
B.

We can observe that two of the carbon atoms have an ether group at one end. But they have only an oxygen atom on the other end, instead of a hydroxyl group. Hence, it does not contain a hemiacetal group.
C.

We can observe that one of the carbon atoms is attached to both an ether and a hydroxyl group. Hence, it does contain a hemiacetal group.
D.

We can observe that one of the carbon atoms has two ether groups at one end. But it has only a benzene molecule on the other end, instead of a hydroxyl group. Hence, it does not contain a hemiacetal group.
Hence, Option C is the correct option
Note: The acetal is derived from a hemiacetal and an alcohol making the second ether group. Hemiacetal Formation: An aldehyde or ketone plus an alcohol may exist in equilibrium with a hemiacetal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




