
Which of the following structures cannot represent resonance form for diamagnetic ${N_2}O$?
A)
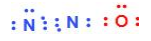
B)

C)

D)

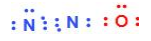



Answer
548.1k+ views
Hint:
To find the structure of a certain molecule, we must recall the electronic configurations of the constituent atoms of the molecule. Valence electrons of an atom are used to form bonds and determine the structure of the molecule. Nitrogen has 5 valence electrons and oxygen has 6 valence electrons.
Complete step by step solution:
In nitrous oxide, the central atom is a nitrogen atom which is bonded to an oxygen atom on one side and to a nitrogen on the other. Resonance involves only the delocalization of electrons. The atoms do not change positions, only the bonding between the atoms changes. Of the given structures, we can see clearly that A and B represent nitrous oxide.
In option C and D, the central atom is given as oxygen which is not the correct representation for nitrous oxide as the position of atoms has been altered.
In option D, the given compound has three nitrogen atoms and no oxygen. Thus it clearly does not represent nitrous oxide.
So, the answers are options C, D and E.
Additional information:
Among nitrogen and oxygen, we know that oxygen is more electronegative than nitrogen. It is preferred that the less electronegative element nitrogen does not carry a negative charge and that resonance structure is more stable and hence more contributing. If we compare option A and B, the structure in option A is more stable resonance structure as in B, the less electronegative atom nitrogen carries a negative charge
Note:
Resonance structures of a molecule describe the delocalization of electrons. They are a set of Lewis structures and are also known as canonical structures.
To find the structure of a certain molecule, we must recall the electronic configurations of the constituent atoms of the molecule. Valence electrons of an atom are used to form bonds and determine the structure of the molecule. Nitrogen has 5 valence electrons and oxygen has 6 valence electrons.
Complete step by step solution:
In nitrous oxide, the central atom is a nitrogen atom which is bonded to an oxygen atom on one side and to a nitrogen on the other. Resonance involves only the delocalization of electrons. The atoms do not change positions, only the bonding between the atoms changes. Of the given structures, we can see clearly that A and B represent nitrous oxide.
In option C and D, the central atom is given as oxygen which is not the correct representation for nitrous oxide as the position of atoms has been altered.
In option D, the given compound has three nitrogen atoms and no oxygen. Thus it clearly does not represent nitrous oxide.
So, the answers are options C, D and E.
Additional information:
Among nitrogen and oxygen, we know that oxygen is more electronegative than nitrogen. It is preferred that the less electronegative element nitrogen does not carry a negative charge and that resonance structure is more stable and hence more contributing. If we compare option A and B, the structure in option A is more stable resonance structure as in B, the less electronegative atom nitrogen carries a negative charge
Note:
Resonance structures of a molecule describe the delocalization of electrons. They are a set of Lewis structures and are also known as canonical structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




