
Which of the following structures are superimposable?
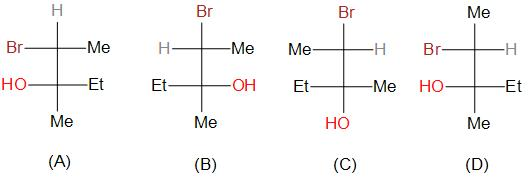
A.A and B
B.B and C
C.A and D
D.A and C

Answer
574.5k+ views
Hint: Two structures are known as superimposable if one structure can be placed on top of the other and look the same. If we make an even number of rotations to the structure, the conformation of the structure remains the same.
Complete step by step solution:
The given structures, that are, A, B, C and D are stereoisomers. The compound has two stereoisomers. Stereoisomers are isomers that differ in spatial arrangement of atoms. Stereoisomers can be of two types, optically active stereoisomers and mesomeric isomers.
The maximum number of stereoisomers possible in a compound can be given by a simple formula,
No. of stereoisomers $ = {2^n}$
Where, $n$ is the number of stereocenters present in the molecule.
In the above question, with the given conditions, the number of stereocenters are two.
As a result, the total number of stereoisomers possible can be given by ${2^2} = 4$
We have with us four stereoisomers in the given question.
If we rotate the structure A, even number of times, we will get the structure C.
Thus we can say that A and C are superimposable structures.
Thus, the correct option is D.
Note:
If the stereoisomers are non- superimposable mirror images of each other, they are said to be optically active.Optical activity is a characteristic of chiral molecules only. We know that a chiral molecule is one that cannot be superimposed on its mirror image. Such a molecule and its non-super imposable mirror image form a pair of enantiomers. Enantiomers rotate the plane of plane polarized light to the same extent but in the opposite direction, that is, one of them is dextrorotatory and the other is laevo rotatory.
Complete step by step solution:
The given structures, that are, A, B, C and D are stereoisomers. The compound has two stereoisomers. Stereoisomers are isomers that differ in spatial arrangement of atoms. Stereoisomers can be of two types, optically active stereoisomers and mesomeric isomers.
The maximum number of stereoisomers possible in a compound can be given by a simple formula,
No. of stereoisomers $ = {2^n}$
Where, $n$ is the number of stereocenters present in the molecule.
In the above question, with the given conditions, the number of stereocenters are two.
As a result, the total number of stereoisomers possible can be given by ${2^2} = 4$
We have with us four stereoisomers in the given question.
If we rotate the structure A, even number of times, we will get the structure C.
Thus we can say that A and C are superimposable structures.
Thus, the correct option is D.
Note:
If the stereoisomers are non- superimposable mirror images of each other, they are said to be optically active.Optical activity is a characteristic of chiral molecules only. We know that a chiral molecule is one that cannot be superimposed on its mirror image. Such a molecule and its non-super imposable mirror image form a pair of enantiomers. Enantiomers rotate the plane of plane polarized light to the same extent but in the opposite direction, that is, one of them is dextrorotatory and the other is laevo rotatory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




