
Which of the following statement(s) is/are correct for \[{\text{PC}}{{\text{l}}_{\text{5}}}\] ? This question has multiple correct options
A. P atom is $sp^3d$-hybridized and has trigonal bipyramidal geometry
B. It acts as a Lewis acid
C. It forms phosphoric acid on hydrolysis
D. Axial Cl atoms are closer to equatorial Cl atoms
Answer
558.3k+ views
Hint: Valence shell electron repulsion theory predicts the geometry of the molecule. The bonding in \[{\text{PC}}{{\text{l}}_{\text{5}}}\] can be understood by VSEPR theory. The shape can be understood by hybridisation.
Complete step by step answer:
\[{\text{PC}}{{\text{l}}_{\text{5}}}\] comprise d orbitals along with s and p orbitals. The energy of the 3d
orbitals are comparable to the energy of 3s as well as 3p orbitals. The energy of 3d orbitals is also comparable to 4s as well as 4p orbitals. As a result, the hybridization of all of these orbitals are feasible.
The five orbitals i.e., 1s, 3p, and 1d orbitals are available for hybridization. Therefore, it can obtain a set of \[{\text{5s}}{{\text{p}}^{\text{3}}}{\text{d}}\] hybrid orbitals. According to VSEPR theory, its shape is trigonal bipyramidal.
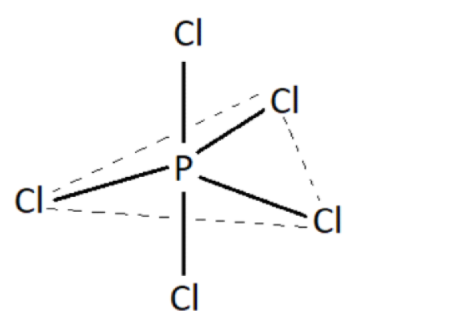
Two type of bonds exist,
A. Equatorial bonds, the \[{\text{3 P-Cl}}\] bond lies in a single plane to make equal angles with each other. The angle made between them is \[{{120^\circ }}\].
B. Axial bonds. Of the remaining \[{\text{2 P-Cl}}\] bonds, one lies above the equatorial plane and the other below the equatorial plane. The angle made with the plane \[{{90^\circ }}\].
The axial bond pairs are more repulsive than the equatorial bond pairs, accounting for this, the axial bonds tend to be slightly longer. Therefore, the axial bonds are slightly weaker than the equatorial bonds.
It can act as a Lewis base because it has the capacity to donate its lone pair of electrons to other electron deficient atoms.
The hydrolysis of phosphorus pentachloride is given by
\[{\text{PC}}{{\text{l}}_{\text{5}}}{\text{ + 4 }}{{\text{H}}_{\text{2}}}{\text{O }} \to {\text{ }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{4 }}}}{\text{ + 5 HCl}}\]
\[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{4 }}}}\] Is phosphoric acid, i.e., it reacts in water to produce phosphoric acid and hydrochloric acid.
So, the correct answer is Option A,B,C.
Additional Information:
\[{\text{PC}}{{\text{l}}_{\text{5}}}\]reacts with sulphur dioxide to form Phosphoryl chloride and Thionyl chloride.
Note: Physically, \[{\text{PC}}{{\text{l}}_{\text{5}}}\] is a pale greenish-yellow solid. It is known to have a salt-like structure in crystalline state and it partly dissociates in solution, especially in polar solvents like nitrobenzene. It is prepared by the action of dry chlorine on phosphorus trichloride.
Complete step by step answer:
\[{\text{PC}}{{\text{l}}_{\text{5}}}\] comprise d orbitals along with s and p orbitals. The energy of the 3d
orbitals are comparable to the energy of 3s as well as 3p orbitals. The energy of 3d orbitals is also comparable to 4s as well as 4p orbitals. As a result, the hybridization of all of these orbitals are feasible.
The five orbitals i.e., 1s, 3p, and 1d orbitals are available for hybridization. Therefore, it can obtain a set of \[{\text{5s}}{{\text{p}}^{\text{3}}}{\text{d}}\] hybrid orbitals. According to VSEPR theory, its shape is trigonal bipyramidal.

Two type of bonds exist,
A. Equatorial bonds, the \[{\text{3 P-Cl}}\] bond lies in a single plane to make equal angles with each other. The angle made between them is \[{{120^\circ }}\].
B. Axial bonds. Of the remaining \[{\text{2 P-Cl}}\] bonds, one lies above the equatorial plane and the other below the equatorial plane. The angle made with the plane \[{{90^\circ }}\].
The axial bond pairs are more repulsive than the equatorial bond pairs, accounting for this, the axial bonds tend to be slightly longer. Therefore, the axial bonds are slightly weaker than the equatorial bonds.
It can act as a Lewis base because it has the capacity to donate its lone pair of electrons to other electron deficient atoms.
The hydrolysis of phosphorus pentachloride is given by
\[{\text{PC}}{{\text{l}}_{\text{5}}}{\text{ + 4 }}{{\text{H}}_{\text{2}}}{\text{O }} \to {\text{ }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{4 }}}}{\text{ + 5 HCl}}\]
\[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{4 }}}}\] Is phosphoric acid, i.e., it reacts in water to produce phosphoric acid and hydrochloric acid.
So, the correct answer is Option A,B,C.
Additional Information:
\[{\text{PC}}{{\text{l}}_{\text{5}}}\]reacts with sulphur dioxide to form Phosphoryl chloride and Thionyl chloride.
Note: Physically, \[{\text{PC}}{{\text{l}}_{\text{5}}}\] is a pale greenish-yellow solid. It is known to have a salt-like structure in crystalline state and it partly dissociates in solution, especially in polar solvents like nitrobenzene. It is prepared by the action of dry chlorine on phosphorus trichloride.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




