
Which of the following statements is true about resonance?
The resonance hybrid explains all features of a molecule.
A.True
B.False
Answer
578.4k+ views
Hint: A resonance hybrid is an intermediate structure of all the contributing structures of a molecule. It tells about the bond characteristics of the molecule as a whole.
Complete step by step answer:
The representation of resonance in benzene is shown above. The equivalent structure shows the delocalization of electrons on the entire ring and is called the resonance hybrid.
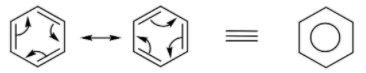
A resonance hybrid represents the delocalization of electrons within the entire molecule.
Of all the resonating structures, the most stable structure contributes the most to the resonance hybrid. The most stable contributing structure has the most number of atoms with complete octets, most number of covalent bonds and least number of formal charges.
The resonance hybrid can only explain some of the bonding features of a molecule and the entire bonding of the molecule cannot be described.
Thus, the given statement is false.
Note:
In a resonance hybrid, all the structures should have the same total number of electrons and the hybridization of the molecule must remain the same. The position of atoms remains unchanged in all the resonating structures, also the number of lone pairs also remains the same. The most stable structure has least separation of formal charge, the most electronegative atom bears the negative charge and the most electropositive atom bears the positive charge.
Complete step by step answer:
The representation of resonance in benzene is shown above. The equivalent structure shows the delocalization of electrons on the entire ring and is called the resonance hybrid.

A resonance hybrid represents the delocalization of electrons within the entire molecule.
Of all the resonating structures, the most stable structure contributes the most to the resonance hybrid. The most stable contributing structure has the most number of atoms with complete octets, most number of covalent bonds and least number of formal charges.
The resonance hybrid can only explain some of the bonding features of a molecule and the entire bonding of the molecule cannot be described.
Thus, the given statement is false.
Note:
In a resonance hybrid, all the structures should have the same total number of electrons and the hybridization of the molecule must remain the same. The position of atoms remains unchanged in all the resonating structures, also the number of lone pairs also remains the same. The most stable structure has least separation of formal charge, the most electronegative atom bears the negative charge and the most electropositive atom bears the positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




