
Which of the following statements is not correct?
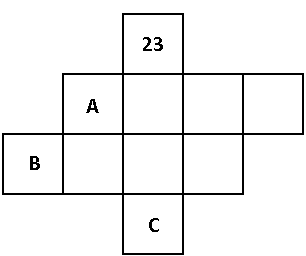
A) Atomic no. of A, B, C are 40, 57, 105 respectively.
B) Group no. of A, B, C are IV B, III B, V B respectively.
C) Period no. of A, B, C are 4th, 5th, 6th respectively.
D) C is a radioactive element.

Answer
563.4k+ views
Hint:To solve this we must know the element having atomic number 23. The element having atomic number 23 is vanadium. The chemical symbol for vanadium is ${\text{V}}$. From this we can now find out the elements A, B and C.
Complete solution:
We know that the element having atomic number 23 is vanadium. Vanadium belongs to period number 4 and group number VB of the periodic table. The chemical symbol for vanadium is ${\text{V}}$.
From this we can conclude that:
The element A belongs to period number 5 and group number IV B of the periodic table. The element A is zirconium. The atomic number of zirconium is 40.The chemical symbol for zirconium is ${\text{Zr}}$.
The element B belongs to period number 6 and group number III B of the periodic table. The element B is lanthanum. The atomic number of lanthanum is 57.The chemical symbol for lanthanum is ${\text{La}}$.
The element C belongs to period number 4 and group number V B of the periodic table. The element C is dubnium. The atomic number of dubnium is 105.The chemical symbol for dubnium is ${\text{Db}}$.
Thus, the statement ‘atomic no. of A, B, C are 40, 57, 105 respectively, is correct. Thus, option (A) is not correct.
Thus, the statement ‘group no. of A, B, C are IV B, III B, V B respectively, is correct. Thus, option (B) is not correct.
Thus, the statement ‘period no. of A, B, C are 4th, 5th, 6th respectively, is not correct. Thus, option (C) is correct.
The element C i.e. dubnium is highly radioactive. It does not occur naturally on earth.
Thus, the statement ‘C is a radioactive element’ is correct. Thus, option (D) is not correct.
Thus, the incorrect statement is period no. of A, B, C are 4th, 5th, 6th respectively.
Thus, the correct option is (C) period no. of A, B, C are 4th, 5th, 6th respectively.
Note: Some elements spontaneously emit particles having high energy like electrons or alpha particles. This property of the elements is known as radioactivity and the elements exhibiting this property are known as radioactive elements.
Complete solution:
We know that the element having atomic number 23 is vanadium. Vanadium belongs to period number 4 and group number VB of the periodic table. The chemical symbol for vanadium is ${\text{V}}$.
From this we can conclude that:
The element A belongs to period number 5 and group number IV B of the periodic table. The element A is zirconium. The atomic number of zirconium is 40.The chemical symbol for zirconium is ${\text{Zr}}$.
The element B belongs to period number 6 and group number III B of the periodic table. The element B is lanthanum. The atomic number of lanthanum is 57.The chemical symbol for lanthanum is ${\text{La}}$.
The element C belongs to period number 4 and group number V B of the periodic table. The element C is dubnium. The atomic number of dubnium is 105.The chemical symbol for dubnium is ${\text{Db}}$.
Thus, the statement ‘atomic no. of A, B, C are 40, 57, 105 respectively, is correct. Thus, option (A) is not correct.
Thus, the statement ‘group no. of A, B, C are IV B, III B, V B respectively, is correct. Thus, option (B) is not correct.
Thus, the statement ‘period no. of A, B, C are 4th, 5th, 6th respectively, is not correct. Thus, option (C) is correct.
The element C i.e. dubnium is highly radioactive. It does not occur naturally on earth.
Thus, the statement ‘C is a radioactive element’ is correct. Thus, option (D) is not correct.
Thus, the incorrect statement is period no. of A, B, C are 4th, 5th, 6th respectively.
Thus, the correct option is (C) period no. of A, B, C are 4th, 5th, 6th respectively.
Note: Some elements spontaneously emit particles having high energy like electrons or alpha particles. This property of the elements is known as radioactivity and the elements exhibiting this property are known as radioactive elements.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




