
Which of the following statements is incorrect for the given graph?
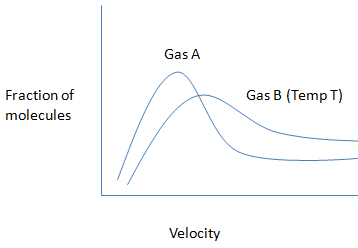
A) The molecular weight of gas A will be greater than gas B
B) The average kinetic energy of both the gases will be similar at a particular temperature.
C) The average velocity of particles of gas A will always be less than of particles of gas B at temperature T.
D) The velocity of any particle of gas A cannot be greater than the velocity of any particle of gas B at the same temperature.

Answer
513.6k+ views
Hint: We have to know that air is one of the important things in our life. In the world, without air nothing is surveyed. For human beings, breathing purpose air is the important thing for inhaling and exhaling. For human respiration, oxygen is used for inhalation of breathing and carbon dioxide is exhaled gas. This inhale and exhale gas are important for human lifestyle to survey in the world. Each and every gas has unique properties and unique nature in the atmosphere. One gas is not able to replace another gas in the environment.
Complete answer:
The given graph is,
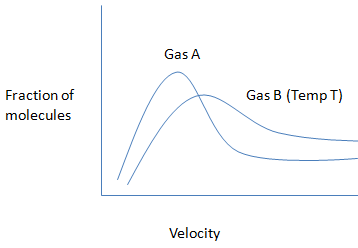
The given statement is
A) The molecular weight of gas A will be greater than gas B
B) The average kinetic energy of both the gases will be similar at a particular temperature.
C) The average velocity of particles of gas A will always be less than of particles of gas B at temperature T.
D) The velocity of any particle of gas A cannot be greater than the velocity of any particle of gas B at the same temperature.
From this we conclude, The molecular weight of gas A will be greater than gas B and the average kinetic energy of both the gases will be not similar at a particular temperature. The average velocity of particles of gas A will always be less than of particles of gas B at temperature T. The velocity of any particle of gas A cannot be greater than the velocity of any particles of gas B at the same temperature.
Hence, option B is an incorrect statement.
Note:
We must have to remember that the chemical properties of the gas depend on the mixture of gases. All the gases are not helpful for the human body. Some gases are poison for the human body. It leads to death also. For example, wrong inhalation of carbon dioxide in the human body leads to death. Some cyanide gases and mono and dioxide gases also lead to human death in the world. The molecular weight of the molecule is dependent on the atomic weight of the atom present in the molecule. The molecular weight of the molecule is equal to the sum of the molecular weight of the atom and the number of the respective atom in the molecule.
${\text{Molecular weight}} = {\text{Number of the atoms}} \times {\text{Atomic weight of the atom}}$
Complete answer:
The given graph is,

The given statement is
A) The molecular weight of gas A will be greater than gas B
B) The average kinetic energy of both the gases will be similar at a particular temperature.
C) The average velocity of particles of gas A will always be less than of particles of gas B at temperature T.
D) The velocity of any particle of gas A cannot be greater than the velocity of any particle of gas B at the same temperature.
From this we conclude, The molecular weight of gas A will be greater than gas B and the average kinetic energy of both the gases will be not similar at a particular temperature. The average velocity of particles of gas A will always be less than of particles of gas B at temperature T. The velocity of any particle of gas A cannot be greater than the velocity of any particles of gas B at the same temperature.
Hence, option B is an incorrect statement.
Note:
We must have to remember that the chemical properties of the gas depend on the mixture of gases. All the gases are not helpful for the human body. Some gases are poison for the human body. It leads to death also. For example, wrong inhalation of carbon dioxide in the human body leads to death. Some cyanide gases and mono and dioxide gases also lead to human death in the world. The molecular weight of the molecule is dependent on the atomic weight of the atom present in the molecule. The molecular weight of the molecule is equal to the sum of the molecular weight of the atom and the number of the respective atom in the molecule.
${\text{Molecular weight}} = {\text{Number of the atoms}} \times {\text{Atomic weight of the atom}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




