
Which of the following species have the bond angle of ${120^\circ }$?
A) $B{F_3}$
B) $PC{l_3}$
C) $Br{F_3}$
D) $N{H_3}$
Answer
570.3k+ views
Hint: As we know that bond angle formed between two bonds originating from the same atom or the angle formed between three atoms across at least two bonds. Bond angles help in distinguishing the geometry of a compound because every compound has different atoms and different geometry.
Complete answer:
As we know that bond angle is an angle between the two bonds of two or more than two atoms. We can discuss the given species or compounds one by one. So first is Boron trifluoride which consists of a central atom of Boron attached with three single bonds of fluorine atoms. As we know boron have atomic number $5$ so it can lose an electron to become stable and fluorine having atomic number $9$ can gain one electron to attain stability. Thus they both can combine with each other and form an angle of ${120^\circ }$. The geometry is generally trigonal planar and fluorine atoms are placed at vertices of an equilateral triangle. Therefore the sharing between the two can be shown as:
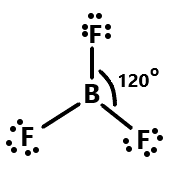
Similarly, the bond angle between phosphorus trichloride can be depicted as the phosphorus having three unpaired electrons so it can share three electrons with chlorine which is having three lone pairs of electrons. The geometry of this compound is trigonal pyramidal with an angle of ${103^\circ }$. We can show this with the help of diagram:
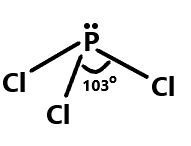
Then comes Bromine trifluoride which has a T-shaped molecular geometry and fluorine atoms are positioned at the ends of T-shaped geometry. The bond angle between the fluorine atoms is approximately ${86^\circ }$. We can show it as:
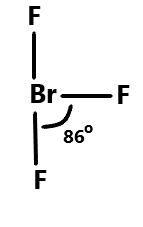
Lastly we have ammonia where the bond angle is reduced to ${107^\circ }$ from ${109.5^\circ }$ because of the repulsion between the lone pair and the bonding pair. It has a trigonal pyramidal shape. We can show it as:
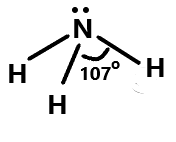
Therefore, from the above explanation the correct answer is (A).
Note: As there are no lone pairs of electrons on the boron in boron trifluoride all the angles will be equal so as to keep the repulsion minimum between the bond pairs of electrons in the compound. All four atoms in this compound lie in the same plane with a simple geometry of trigonal planar.
Complete answer:
As we know that bond angle is an angle between the two bonds of two or more than two atoms. We can discuss the given species or compounds one by one. So first is Boron trifluoride which consists of a central atom of Boron attached with three single bonds of fluorine atoms. As we know boron have atomic number $5$ so it can lose an electron to become stable and fluorine having atomic number $9$ can gain one electron to attain stability. Thus they both can combine with each other and form an angle of ${120^\circ }$. The geometry is generally trigonal planar and fluorine atoms are placed at vertices of an equilateral triangle. Therefore the sharing between the two can be shown as:

Similarly, the bond angle between phosphorus trichloride can be depicted as the phosphorus having three unpaired electrons so it can share three electrons with chlorine which is having three lone pairs of electrons. The geometry of this compound is trigonal pyramidal with an angle of ${103^\circ }$. We can show this with the help of diagram:

Then comes Bromine trifluoride which has a T-shaped molecular geometry and fluorine atoms are positioned at the ends of T-shaped geometry. The bond angle between the fluorine atoms is approximately ${86^\circ }$. We can show it as:

Lastly we have ammonia where the bond angle is reduced to ${107^\circ }$ from ${109.5^\circ }$ because of the repulsion between the lone pair and the bonding pair. It has a trigonal pyramidal shape. We can show it as:

Therefore, from the above explanation the correct answer is (A).
Note: As there are no lone pairs of electrons on the boron in boron trifluoride all the angles will be equal so as to keep the repulsion minimum between the bond pairs of electrons in the compound. All four atoms in this compound lie in the same plane with a simple geometry of trigonal planar.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




