
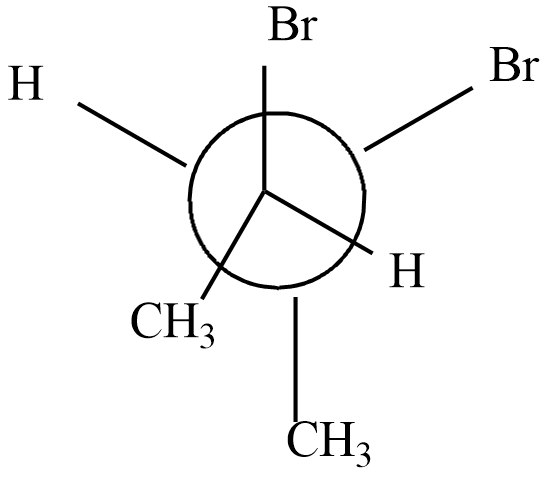
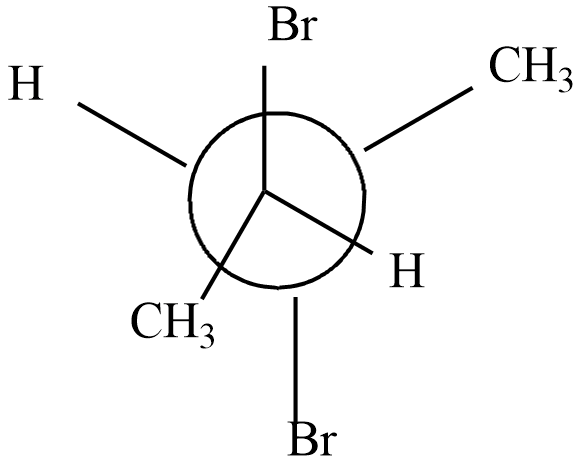
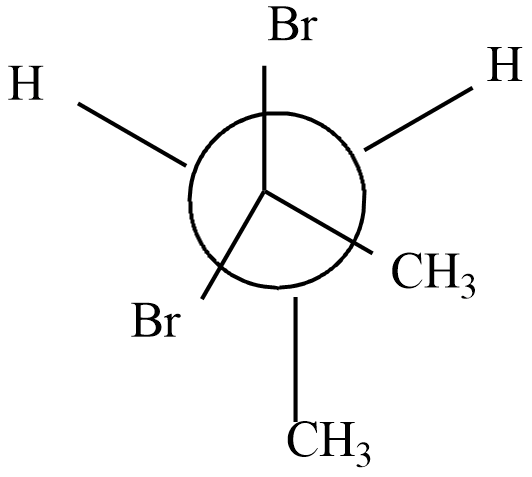
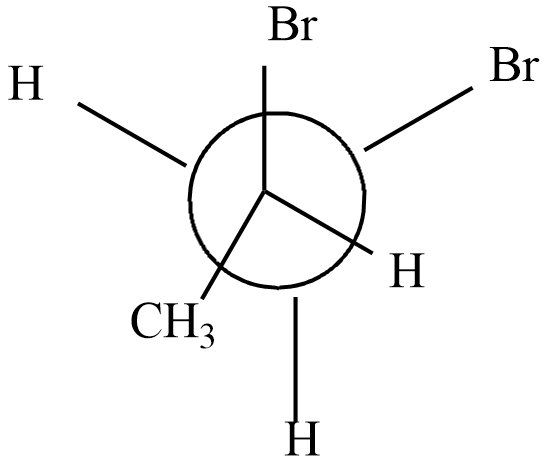
Which of the following represents the meso form of $ 2,3 - dibromobu\tan e $
a)
b)
c)
d)




Answer
546.3k+ views
Hint :Meso compounds are those which either have an internal plane of symmetry or they can have a centre of symmetry inside the structure of the molecule. Conversion of Newman to sawhorse and then to Fischer projection is necessary.
Complete Step By Step Answer:
Chiral carbons are those carbons which have four different substituent groups. These types of carbon centres cause optical nature in some compounds.
A meso compound can be termed as the molecule containing chiral centre and optically inactive due to an internal plane of symmetry and a centre of symmetry. It must have at least two chiral centres.
In meso compounds the plane divides the molecule into halves which are mirror images of each other. This is also known as internal compensation causing optical inactivity of the compound.
The number of chiral centres can be calculated by checking the number of carbon in the compound having four different substituents attached to it. So the stereoisomers can be calculated by the formula given as -
$ {2^n} $ Where $ n $ is the number of chiral centres.
In the given molecule $ 2,3 - dibromobu\tan e $ , the number of chiral carbon can be calculated as two
$ \begin{gathered}
\therefore n = 2 \\
\Rightarrow {2^n} = {2^2} = 4 \\
\end{gathered} $
Thus the number of stereoisomers is four. Out of these four two compounds are represented as pairs of enantiomers. And the other two are meso compounds.
The Fischer projection can be converted into Newman by shrinking the two middle carbons and placing the atoms according to their position mentioned in Fischer. The element on the RHS on the vertical line should be on RHS and the element on LHS on the vertical line will be on LHS. The top and bottom will have the first and last groups on the vertical line of Fischer projection.
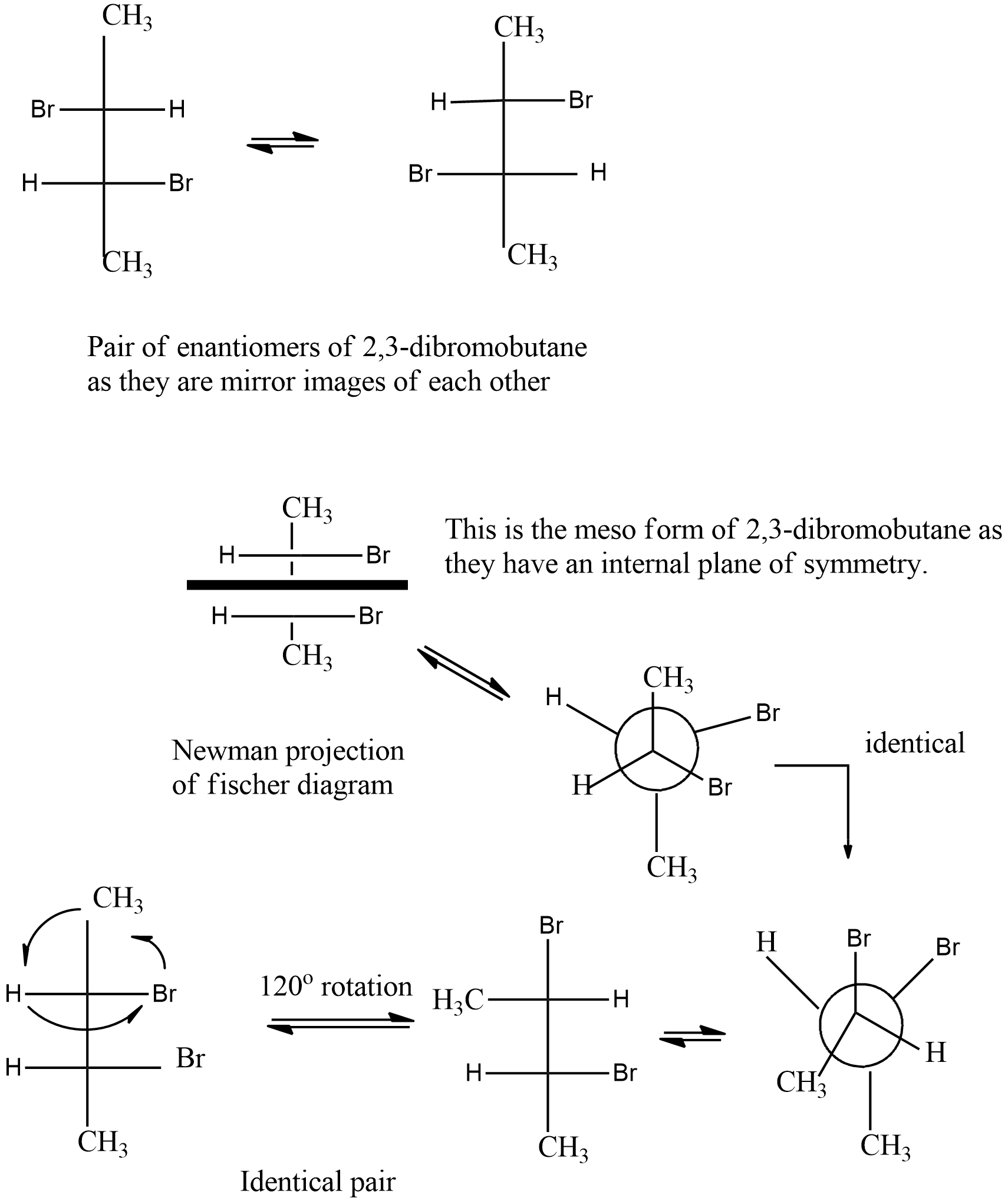
Hence option (a) is correct.
Note :
To be in the criteria of meso compound it is necessary to have at least two chiral centres. Also meso compounds do not have optical activity. But when calculating stereoisomers, meso compounds are also taken into account.
Complete Step By Step Answer:
Chiral carbons are those carbons which have four different substituent groups. These types of carbon centres cause optical nature in some compounds.
A meso compound can be termed as the molecule containing chiral centre and optically inactive due to an internal plane of symmetry and a centre of symmetry. It must have at least two chiral centres.
In meso compounds the plane divides the molecule into halves which are mirror images of each other. This is also known as internal compensation causing optical inactivity of the compound.
The number of chiral centres can be calculated by checking the number of carbon in the compound having four different substituents attached to it. So the stereoisomers can be calculated by the formula given as -
$ {2^n} $ Where $ n $ is the number of chiral centres.
In the given molecule $ 2,3 - dibromobu\tan e $ , the number of chiral carbon can be calculated as two
$ \begin{gathered}
\therefore n = 2 \\
\Rightarrow {2^n} = {2^2} = 4 \\
\end{gathered} $
Thus the number of stereoisomers is four. Out of these four two compounds are represented as pairs of enantiomers. And the other two are meso compounds.
The Fischer projection can be converted into Newman by shrinking the two middle carbons and placing the atoms according to their position mentioned in Fischer. The element on the RHS on the vertical line should be on RHS and the element on LHS on the vertical line will be on LHS. The top and bottom will have the first and last groups on the vertical line of Fischer projection.

Hence option (a) is correct.
Note :
To be in the criteria of meso compound it is necessary to have at least two chiral centres. Also meso compounds do not have optical activity. But when calculating stereoisomers, meso compounds are also taken into account.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




