
Which of the following represents physical adsorption?
A.
B.
C.
D.




Answer
600.9k+ views
Physical adsorption is a type of adsorption that occurs for most particles in contact with a solid or liquid surface. It is a technique that is used to characterize the surface and pore features of solids.
Complete step by step answer:
Adsorption is referred to as the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solids to the surface. This process creates a film of the adsorbate on the surface of the adsorbent.
Physical adsorption is the condensation of gases to liquids. It depends on the Van der Waal force of attraction between the solid adsorbent and the adsorbate molecules.
A physically adsorbed molecule can be removed as it is at reduced pressure at the same temperature where the adsorption took place.
It can involve the formation of multimolecular layers.
Physical adsorption is instantaneous.
Physical adsorption will occur at a suitable temperature-pressure condition. Any gas can be adsorbed on any solid if the temperature is sufficiently low or the pressure of the gas is sufficiently high.
The heat that is released from the adsorption does not exceed 5 kcal/mol.
The physical adsorption increases with the increases in gas pressure. And it decreases with the increases in temperature.
The graphs given in the option indicate the relation between the amount of adsorption and the temperature.
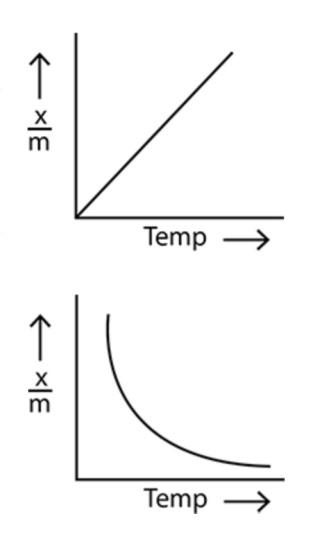
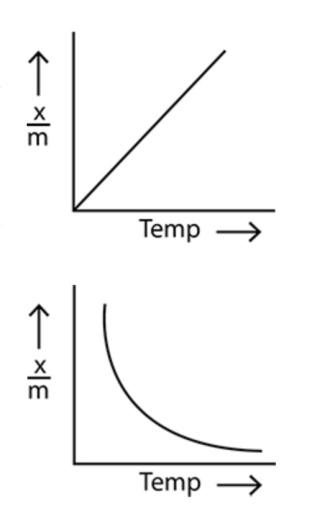
In option (a), the amount of adsorption decreases exponentially with the increase in temperature.
In option (b), the amount of adsorption increases linearly with the increase in temperature.
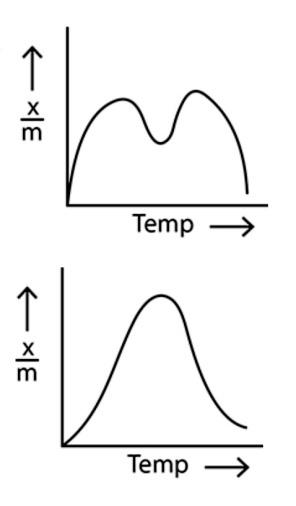
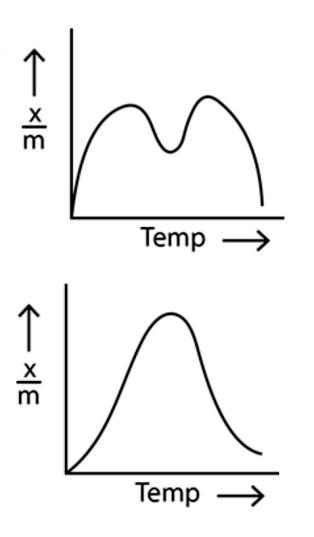
In option (c), the amount of adsorption first increases with the increase in temperature and then decreases with the increase in temperature.
In the last option, option (d), the relation between the amount of adsorption and temperature is a non linear graph with various ups and downs.
Now, considering the point that the amount of adsorption decreases with the increase in temperature, we can see that only the option (a) shows the correct relationship between the amount of physical adsorption and the temperature. Hence, option (a) is the correct option.
Note: Students tend to confuse between adsorption and absorption. These two are completely different things. Adsorption is referred to as the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to the surface. Whereas, absorption is a bulk phenomenon where the absorbance enters into the absorbent.
Complete step by step answer:
Adsorption is referred to as the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solids to the surface. This process creates a film of the adsorbate on the surface of the adsorbent.
Physical adsorption is the condensation of gases to liquids. It depends on the Van der Waal force of attraction between the solid adsorbent and the adsorbate molecules.
A physically adsorbed molecule can be removed as it is at reduced pressure at the same temperature where the adsorption took place.
It can involve the formation of multimolecular layers.
Physical adsorption is instantaneous.
Physical adsorption will occur at a suitable temperature-pressure condition. Any gas can be adsorbed on any solid if the temperature is sufficiently low or the pressure of the gas is sufficiently high.
The heat that is released from the adsorption does not exceed 5 kcal/mol.
The physical adsorption increases with the increases in gas pressure. And it decreases with the increases in temperature.
The graphs given in the option indicate the relation between the amount of adsorption and the temperature.
In option (a), the amount of adsorption decreases exponentially with the increase in temperature.
In option (b), the amount of adsorption increases linearly with the increase in temperature.
In option (c), the amount of adsorption first increases with the increase in temperature and then decreases with the increase in temperature.
In the last option, option (d), the relation between the amount of adsorption and temperature is a non linear graph with various ups and downs.
Now, considering the point that the amount of adsorption decreases with the increase in temperature, we can see that only the option (a) shows the correct relationship between the amount of physical adsorption and the temperature. Hence, option (a) is the correct option.
Note: Students tend to confuse between adsorption and absorption. These two are completely different things. Adsorption is referred to as the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to the surface. Whereas, absorption is a bulk phenomenon where the absorbance enters into the absorbent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




