
Which of the following represent correctly the changes in thermodynamic properties during the formation of 1 mol of ideal binary solution.
A.
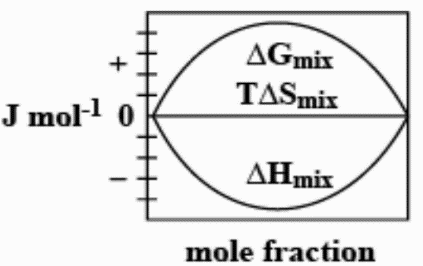
B.
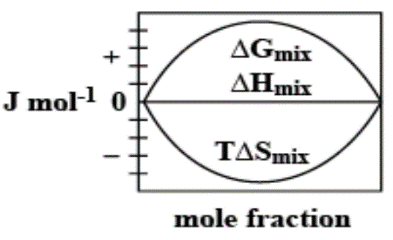
C.
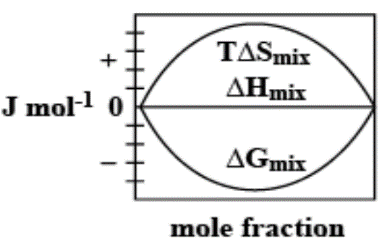
D.
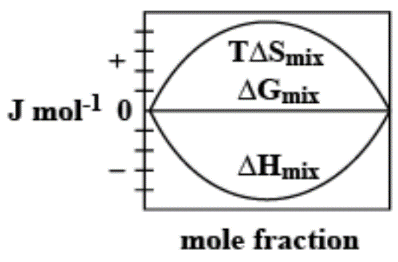




Answer
564.9k+ views
Hint: An ideal mixture is a solution in which the gas exhibits thermodynamic properties analogous to those of a mixture of an ideal gas. Mole fraction is defined as a unit of the amount of a constituent, divided by the total amount of all constituents in a mixture. A mixture of known mole fraction can be prepared by weighing off the appropriate masses of the constituents. In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to the total pressure of the mixture.
Complete step by step answer:
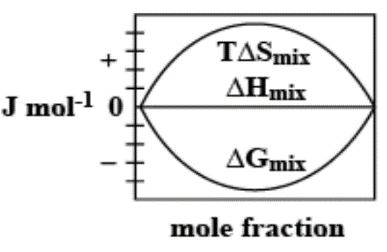
The enthalpy of mixing of 1 mole of ideal binary $0Jmo{l^{ - 1}}0$. It is known that as the mole fraction increases, the free energy change of mixing initially decreases, and it reaches a minimum value and then increases. Furthermore, with an increase in the mole fraction, the value of $T\Delta {S_{mix}}$increases and increases and finally lowers.
Thus, option C. is the right answer.
The following diagram correctly represents the changes in the thermodynamic properties during the formation of 1 mol of ideal binary solution.
Note:
The thermodynamic properties are volume, enthalpy, and heat capacity, the entropy of mixing. If entropies are known separately for the reactants and products, then the change in entropy is just the difference and in the same way the other thermodynamic functions. The mole fraction is also called an amount fraction. It is identical to the number fraction, which is defined as the number of molecules of a constituent. The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity, mass fraction and volume fraction.
Complete step by step answer:
The enthalpy of mixing of 1 mole of ideal binary $0Jmo{l^{ - 1}}0$. It is known that as the mole fraction increases, the free energy change of mixing initially decreases, and it reaches a minimum value and then increases. Furthermore, with an increase in the mole fraction, the value of $T\Delta {S_{mix}}$increases and increases and finally lowers.
Thus, option C. is the right answer.
The following diagram correctly represents the changes in the thermodynamic properties during the formation of 1 mol of ideal binary solution.

Note:
The thermodynamic properties are volume, enthalpy, and heat capacity, the entropy of mixing. If entropies are known separately for the reactants and products, then the change in entropy is just the difference and in the same way the other thermodynamic functions. The mole fraction is also called an amount fraction. It is identical to the number fraction, which is defined as the number of molecules of a constituent. The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity, mass fraction and volume fraction.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




