
Which of the following reactions would give $ trans - $ alkene.
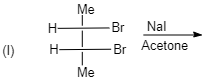
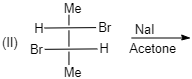
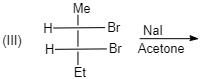
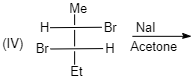
A. (I)
B. (II)
C. (I), (III)
D. (II), (IV)




Answer
508.2k+ views
Hint :An optically inactive molecule whose molecule is superimposable on its mirror image in spite of the presence of asymmetric carbon atoms is known as meso compound. Such a molecule can be recognized by the fact that it possesses a mirror plane which divides the molecule into two halves which are mirror images of each other. Optical inactivity of a meso compound is due to internal compensation.
Complete Step By Step Answer:
$ cis $ -form indicates that the substituents are on the same side of some plane (adjacent positions) and Trans-form indicates that the substituents are on the opposite positions.
Meso compounds having two same groups’ present with Anti-elimination gives $ trans $ -alkene.
Racemic compound having two same groups in Anti-elimination gives $ cis $ -alkene.
Racemic compounds with two different groups but the two eliminating groups present in anti-position on anti-elimination gives Trans-alkene as a product.
Racemic compound with two different groups but the two eliminating groups present in syn-position on anti-elimination gives $ cis $ -alkene.
(I)
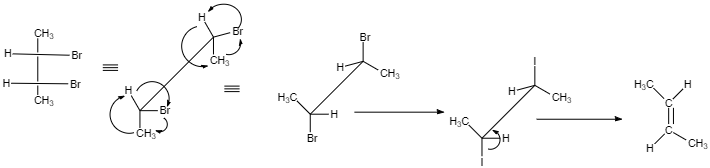
(II)
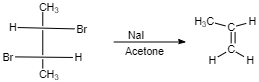
(III)
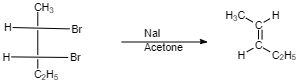
(IV)
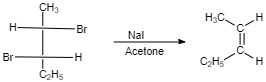
Therefore the correct answer is option C.
Note :
When there are three or four different groups attached to the carbon atoms of a double bond, it becomes difficult to assign $ cis $ and $ trans $ designation to isomers, to overcome this problem, a general system of geometrical isomers called the E and Z system was framed.
If the two higher prioritized groups are on the same side of the double bond, the isomer is called Z and if the two higher prioritized groups are on opposite sides of the double bond, the isomer is called E.
Complete Step By Step Answer:
$ cis $ -form indicates that the substituents are on the same side of some plane (adjacent positions) and Trans-form indicates that the substituents are on the opposite positions.
Meso compounds having two same groups’ present with Anti-elimination gives $ trans $ -alkene.
Racemic compound having two same groups in Anti-elimination gives $ cis $ -alkene.
Racemic compounds with two different groups but the two eliminating groups present in anti-position on anti-elimination gives Trans-alkene as a product.
Racemic compound with two different groups but the two eliminating groups present in syn-position on anti-elimination gives $ cis $ -alkene.
(I)

(II)

(III)

(IV)

Therefore the correct answer is option C.
Note :
When there are three or four different groups attached to the carbon atoms of a double bond, it becomes difficult to assign $ cis $ and $ trans $ designation to isomers, to overcome this problem, a general system of geometrical isomers called the E and Z system was framed.
If the two higher prioritized groups are on the same side of the double bond, the isomer is called Z and if the two higher prioritized groups are on opposite sides of the double bond, the isomer is called E.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




