
Which of the following reactions will give benzophenone?
(i) Benzoyl chloride + Benzene + \[AlC{{l}_{3}}\]
(ii) Benzoyl chloride + Phenylmagnesium bromide
(iii) Benzoyl chloride + Diphenyl cadmium
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (i), (ii) and (iii)
Answer
522.3k+ views
Hint: Benzophenone is a colourless crystalline solid with a rose-like smell. It is practically insoluble in water, but readily soluble in organic solvents such as alcohol, acetone, ether, acetic acid, chloroform, and benzene.
Complete step by step solution:
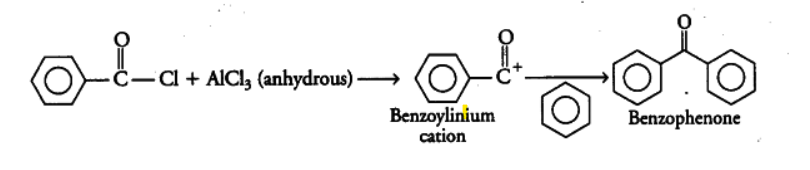
(i) Upon reacting with an excess of benzene in the presence of \[AlC{{l}_{3}}\], benzoyl chloride will give benzophenone by replacing the chlorine with the benzene. It will undergo a reaction called Friedel-Craft’s acylation. It forms an intermediate called benzoylinium cation. The reaction can be given as:
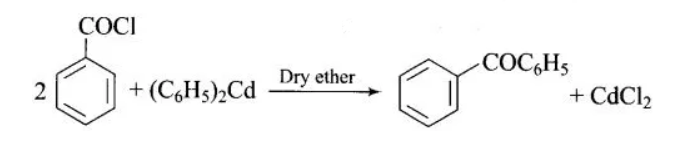
(iii) Similar to the above reaction, when benzoyl chloride reacts with diphenyl cadmium in the presence of dry ether, it replaces the chlorine to form benzophenone. In this reaction, cadmium chloride is formed as a byproduct. The reaction is given as:
So, the correct option is (c).
Additional Information:
Benzophenone is commonly used as a flavor ingredient, a fragrance enhancer, a perfume fixative. It is also used in the manufacturing of agricultural chemicals, insecticides, hypnotic drugs, and other pharmaceuticals.
Benzophenone is used to filter ultraviolet (UV) rays in sunglasses and to prevent UV light from damaging scents and colours in products such as perfumes and soaps.
Benzophenone is also a very good wetting agent for pigments; which can be used in printing to improve the rheological properties and increase the fluidity of inks by acting as a reactive solvent.
Note: An effective sixty six percent yield of benzophenone can be obtained by Friedel-Crafts acylation of benzoyl chloride with an excess of benzene in the presence of anhydrous aluminum chloride.
Complete step by step solution:
(i) Upon reacting with an excess of benzene in the presence of \[AlC{{l}_{3}}\], benzoyl chloride will give benzophenone by replacing the chlorine with the benzene. It will undergo a reaction called Friedel-Craft’s acylation. It forms an intermediate called benzoylinium cation. The reaction can be given as:

(iii) Similar to the above reaction, when benzoyl chloride reacts with diphenyl cadmium in the presence of dry ether, it replaces the chlorine to form benzophenone. In this reaction, cadmium chloride is formed as a byproduct. The reaction is given as:

So, the correct option is (c).
Additional Information:
Benzophenone is commonly used as a flavor ingredient, a fragrance enhancer, a perfume fixative. It is also used in the manufacturing of agricultural chemicals, insecticides, hypnotic drugs, and other pharmaceuticals.
Benzophenone is used to filter ultraviolet (UV) rays in sunglasses and to prevent UV light from damaging scents and colours in products such as perfumes and soaps.
Benzophenone is also a very good wetting agent for pigments; which can be used in printing to improve the rheological properties and increase the fluidity of inks by acting as a reactive solvent.
Note: An effective sixty six percent yield of benzophenone can be obtained by Friedel-Crafts acylation of benzoyl chloride with an excess of benzene in the presence of anhydrous aluminum chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




