
Which of the following reactions products is correct, correspondingly?
A.
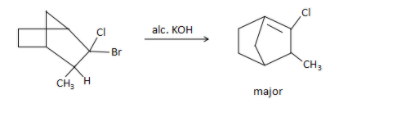
B.
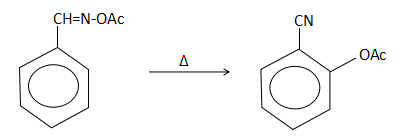
C.
D. $CH \equiv CH\xrightarrow{{\Delta ,C{H_2}{N_2}}}\square $



Answer
578.7k+ views
Hint: An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the \[{E_2}\] reaction, and the two-step mechanism is known as the \[{E_1}\] reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: \[{E_2}\] is bimolecular (second-order) while \[{E_1}\] is unimolecular (first-order).
Complete answer:
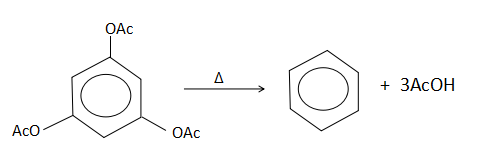
From the above mentioned reactions, all the reactions take place under the condition of applied heat. This causes an elimination reaction to take place. But in the first and second option, although there is an elimination taking place in the presence of heat, the product that is formed is not correct. In the last option, it is given that no product is formed which is wrong. It will form a hydrazine like compound as the product. This leaves us with the third option which is true. When heat is supplied to 1,3,5- trimethoxybenzene, three moles of acetic acid is lost from the substrate molecule to give benzene as the product.
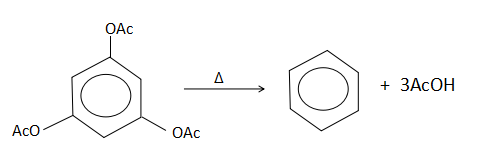 .
.
Thus, the correct option is C.
Note:
The above process is employed in the industries on a large scale for the production of the acetic acid and it is also a good example of the elimination reaction of first order or unimolecular elimination reaction. In most of the organic elimination reactions, at least one hydrogen (\[{H^ + }\]) is lost to form the double bond: the unsaturation of the molecule increases.
Complete answer:
From the above mentioned reactions, all the reactions take place under the condition of applied heat. This causes an elimination reaction to take place. But in the first and second option, although there is an elimination taking place in the presence of heat, the product that is formed is not correct. In the last option, it is given that no product is formed which is wrong. It will form a hydrazine like compound as the product. This leaves us with the third option which is true. When heat is supplied to 1,3,5- trimethoxybenzene, three moles of acetic acid is lost from the substrate molecule to give benzene as the product.

Thus, the correct option is C.
Note:
The above process is employed in the industries on a large scale for the production of the acetic acid and it is also a good example of the elimination reaction of first order or unimolecular elimination reaction. In most of the organic elimination reactions, at least one hydrogen (\[{H^ + }\]) is lost to form the double bond: the unsaturation of the molecule increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




