
Which of the following reactions do not present in the major product of the given Birch reductions?
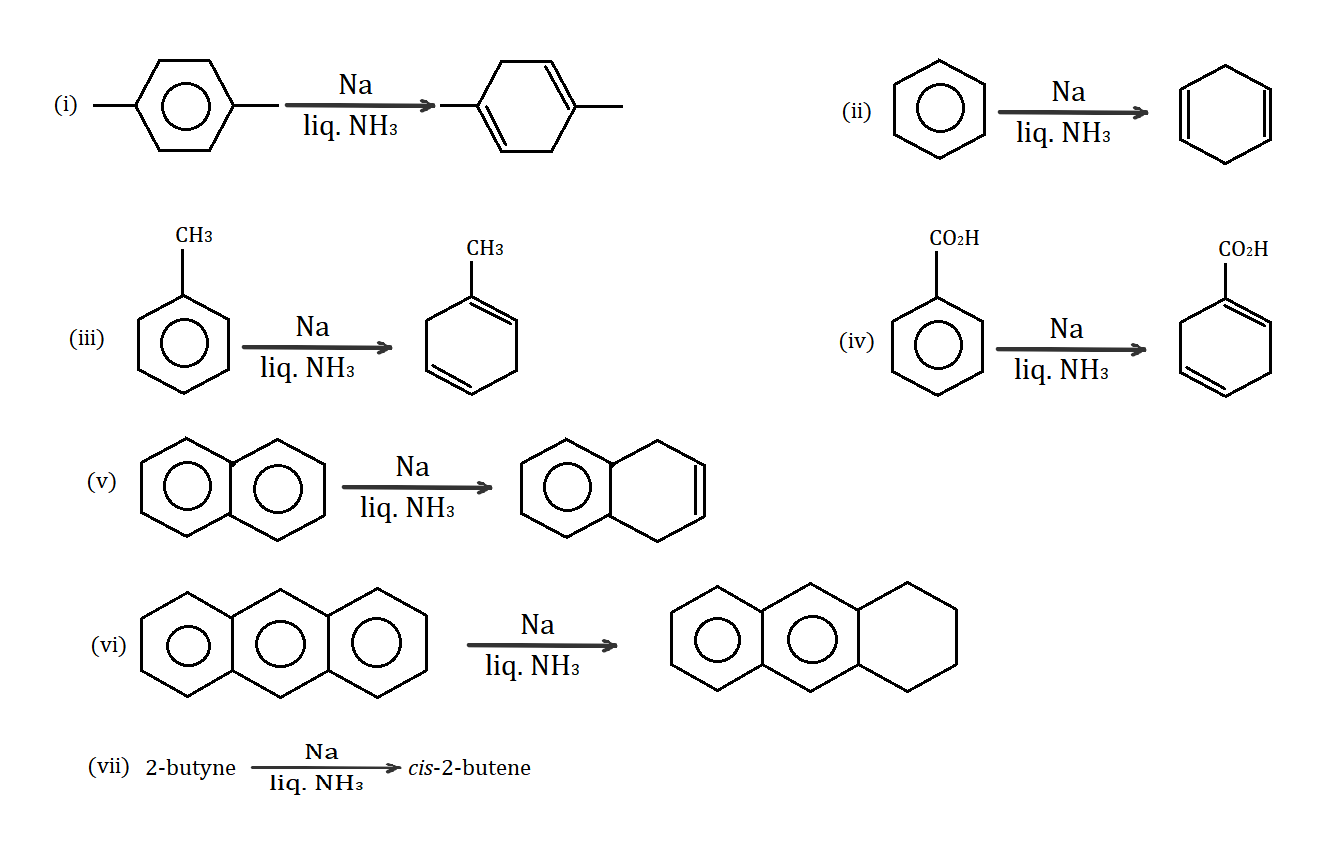
(A) (i), (iii), (iv)
(B) (iv), (vi), (vii)
(C) (iv), (v), (vi)
(D) (i), (ii), (v), (vii)

Answer
524.4k+ views
Hint :The Birch Reduction is the process in which benzene (and its aromatic relatives) is converted to $ 1,4 - $ cyclohexadiene using sodium (or lithium) as a reducing agent in liquid ammonia as solvent (boiling point: $ - 33^\circ C $ ) in the presence of an alcohol such as ethanol, methanol or t-butanol. Here, $ Na $ acts as a reducing agent in the presence of liquid $ N{H_3} $ .
Complete Step By Step Answer:
Reaction (i) satisfies the Birch reduction as there is a formation of $ 1,4 - $ cyclohexadiene ring from a benzene ring.
Reaction (ii) also satisfies the Birch reduction as benzene is simply converted to $ 1,4 - $ cyclohexadiene.
Reaction (iii) also satisfies the Birch reduction as Toluene (an aromatic compound) is converted to $ 1 - $ methyl $ - 1,4 - $ cyclohexadiene.
Reaction (iv) does not satisfy the Birch reduction. In Benzoic acid, $ COOH $ is an electron withdrawing group. On treatment with $ Na $ in the presence of $ N{H_3} $ , it results in the protonation of carbon which has the electron withdrawing group, like $ COOH $ , where the electron is transferred to oxygen (which is more stable) due to regioselectivity. However, in birch reduction, only the phenyl group needs to be protonated and not the functional groups.
Reactive (v) satisfies the birch reduction. Here, naphthalene gets converted into $ 1,4 - $ dihydronaphthalene.
Reactive (vi) does not satisfy the birch reduction. Here, anthracene only loses one double bond and forms conjugate to become $ 9,10 - $ dihydroanthracene.
In reaction (vii), neither the reactants nor the products have a benzene ring or any other aromatic compound. It does not qualify for birch reduction.
Hence, the correct option is B.
Note :
You should be clear that birch reduction occurs only with $ Na/N{H_3} $ . It should not be confused with $ NaN{H_2}/N{H_3} $ . Also, you might sometimes replace $ C - H $ with anyne, $ N{H_2} $ which is again incorrect.
Complete Step By Step Answer:
Reaction (i) satisfies the Birch reduction as there is a formation of $ 1,4 - $ cyclohexadiene ring from a benzene ring.
Reaction (ii) also satisfies the Birch reduction as benzene is simply converted to $ 1,4 - $ cyclohexadiene.
Reaction (iii) also satisfies the Birch reduction as Toluene (an aromatic compound) is converted to $ 1 - $ methyl $ - 1,4 - $ cyclohexadiene.
Reaction (iv) does not satisfy the Birch reduction. In Benzoic acid, $ COOH $ is an electron withdrawing group. On treatment with $ Na $ in the presence of $ N{H_3} $ , it results in the protonation of carbon which has the electron withdrawing group, like $ COOH $ , where the electron is transferred to oxygen (which is more stable) due to regioselectivity. However, in birch reduction, only the phenyl group needs to be protonated and not the functional groups.
Reactive (v) satisfies the birch reduction. Here, naphthalene gets converted into $ 1,4 - $ dihydronaphthalene.
Reactive (vi) does not satisfy the birch reduction. Here, anthracene only loses one double bond and forms conjugate to become $ 9,10 - $ dihydroanthracene.
In reaction (vii), neither the reactants nor the products have a benzene ring or any other aromatic compound. It does not qualify for birch reduction.
Hence, the correct option is B.
Note :
You should be clear that birch reduction occurs only with $ Na/N{H_3} $ . It should not be confused with $ NaN{H_2}/N{H_3} $ . Also, you might sometimes replace $ C - H $ with anyne, $ N{H_2} $ which is again incorrect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




