
Which of the following pairs of compounds are position isomers?
A.Isobutyl alcohol and s-butyl alcohol
B.Isobutyl alcohol and t-butyl alcohol
C.Isobutyl alcohol and neo-pentyl alcohol
D.Ethyl alcohol and ethylene glycol
Answer
512.1k+ views
Hint: We have to know that isomers are two atoms with a similar subatomic recipe however vary fundamentally. In this manner, isomers contain a similar number of iotas for every component, except the nuclear game plan varies. In spite of having a similar subatomic equation, the actual properties of every particle may vary, especially if the practical gatherings related with every atom are unique.
Complete answer:
We have to know that the position of isomers depends on the development of a 'utilitarian gathering' in the atom. A utilitarian gathering in natural science is the piece of a particle that gives it its reactivity. There are a scope of various useful gatherings, the more normal of which were summed up in a past post here. Nothing else about the particle changes basically, where the useful gathering in it is, and the name essentially adjusts somewhat to demonstrate whereabouts in the atom it is found.
For Isobutyl alcohol and s-butyl alcohol, it does not follow the above position of isomers.
Therefore, the option (A) is incorrect.
For Isobutyl alcohol and t-butyl alcohol, it follows the above position isomers.
The structure of the compounds drawn,
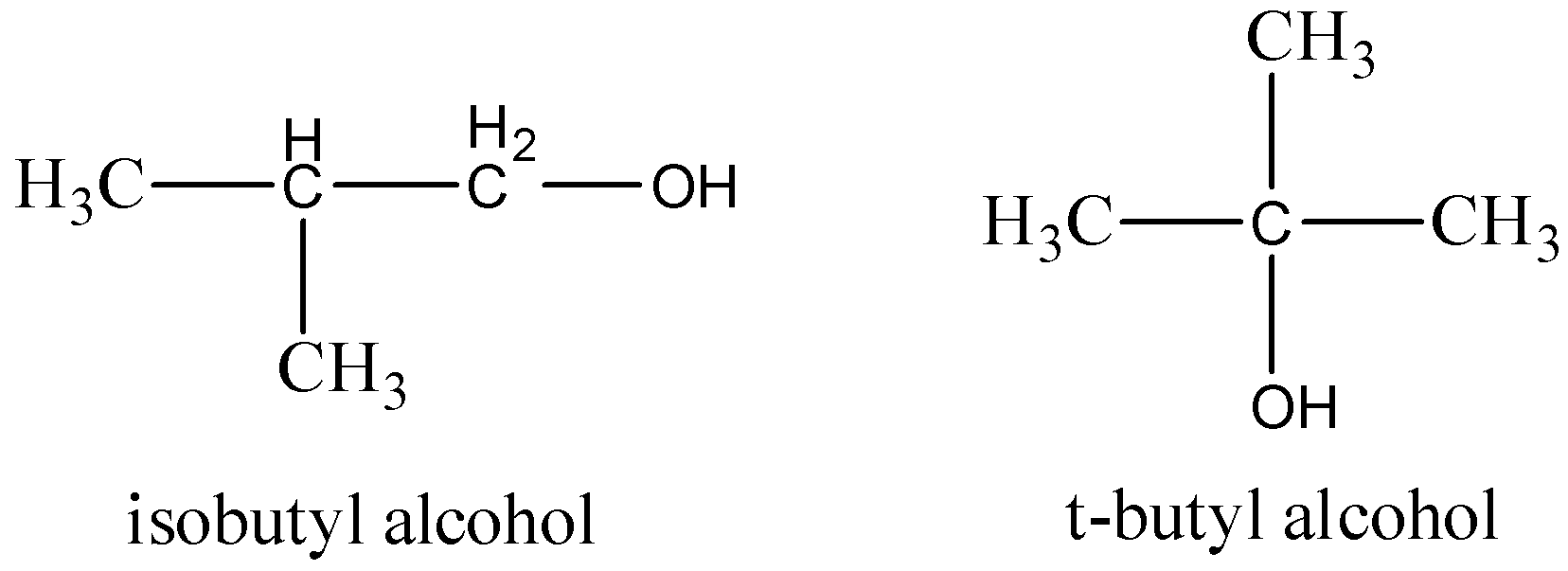
Therefore, the option (B) is correct.
For Isobutyl alcohol and neo-pentyl alcohol, it does not follow the above position of isomers.
Therefore, the option (C) is incorrect.
For ethyl alcohol and ethylene glycol, it does not follow the above position isomers.
Therefore, the option (D) is incorrect.
Note:
We have to know that, the two isomeric mixtures, made out of the very same components in the very same proportion, may have totally different properties in light of the various ways that the molecules are associated inside the mixtures.
Complete answer:
We have to know that the position of isomers depends on the development of a 'utilitarian gathering' in the atom. A utilitarian gathering in natural science is the piece of a particle that gives it its reactivity. There are a scope of various useful gatherings, the more normal of which were summed up in a past post here. Nothing else about the particle changes basically, where the useful gathering in it is, and the name essentially adjusts somewhat to demonstrate whereabouts in the atom it is found.
For Isobutyl alcohol and s-butyl alcohol, it does not follow the above position of isomers.
Therefore, the option (A) is incorrect.
For Isobutyl alcohol and t-butyl alcohol, it follows the above position isomers.
The structure of the compounds drawn,
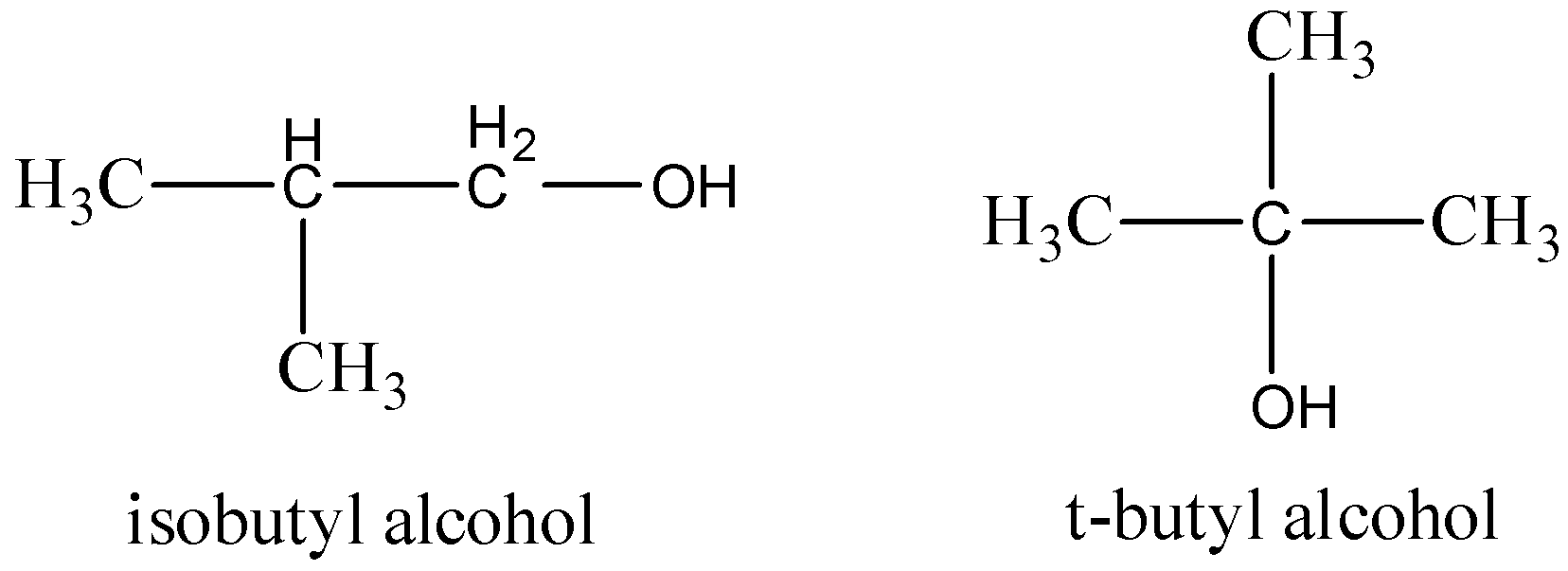
Therefore, the option (B) is correct.
For Isobutyl alcohol and neo-pentyl alcohol, it does not follow the above position of isomers.
Therefore, the option (C) is incorrect.
For ethyl alcohol and ethylene glycol, it does not follow the above position isomers.
Therefore, the option (D) is incorrect.
Note:
We have to know that, the two isomeric mixtures, made out of the very same components in the very same proportion, may have totally different properties in light of the various ways that the molecules are associated inside the mixtures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




