
Which of the following overlaps is not present in $Xe{{O}_{3}}$ molecule?
A. $s{{p}^{3}}+{{p}_{x}}$
B. $s{{p}^{3}}+{{p}_{y}}$
C. ${{d}_{xz}}+{{p}_{x}}$
D. $s{{p}^{3}}+s$
Answer
578.4k+ views
Hint: Xenon forms two types of oxides. They are xenon trioxide and xenon tetroxide. The hybridization of xenon trioxide ($Xe{{O}_{3}}$ ) is $s{{p}^{3}}$ ). We have to draw an orbital diagram to know the overlapping of orbitals in the formation of xenon trioxide.
Complete answer:
- In the question it is asked to find which overlapping among the given options is not present in the xenon trioxide.
- Xenon belongs to group VIII A in the periodic table.
- Oxygen belongs to group VI A in the periodic table.
- The orbital overlapping and hybridization of the oxygen atoms with the xenon atom is as follows.
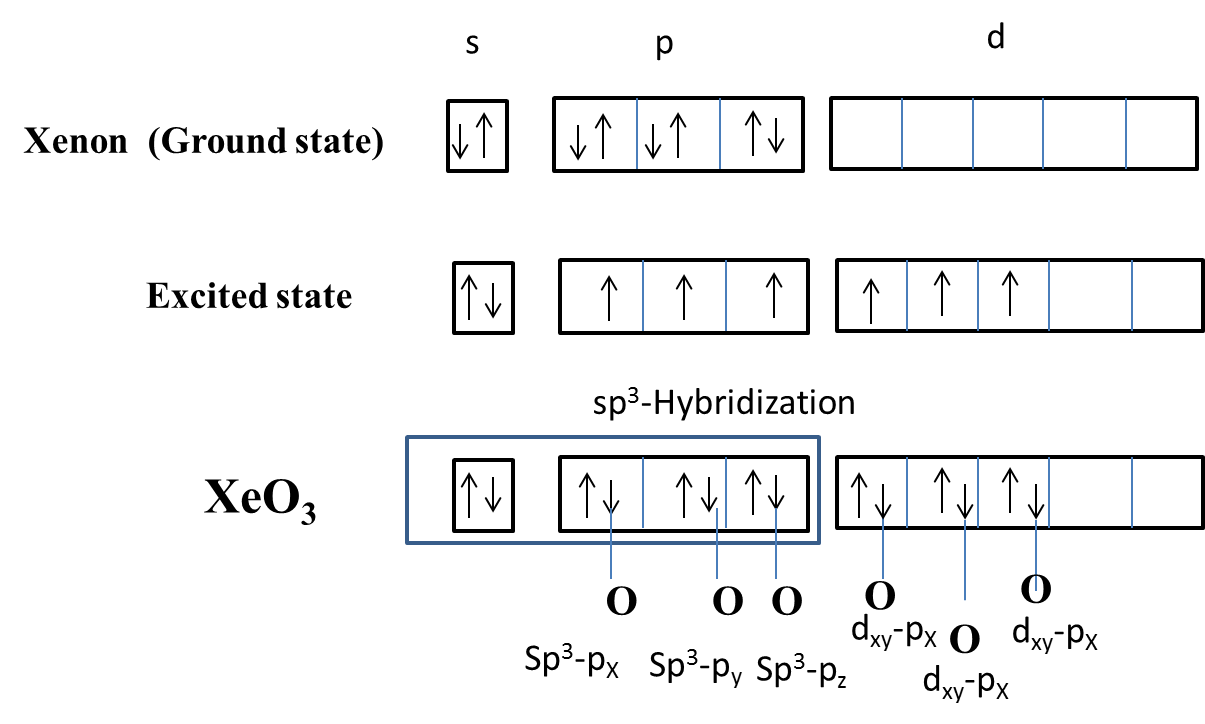
- From the above picture we can say that $s{{p}^{3}}+{{p}_{x}}$ , $s{{p}^{3}}+{{p}_{y}}$ and $s{{p}^{3}}+{{p}_{z}}$ are involved in the hybridization of xenon trioxide.
- Coming to option C, ${{d}_{xz}}+{{p}_{x}}$ is also involved in the hybridization process of xenon trioxide.
- We cannot see any $s{{p}^{3}}+s$ type of overlapping in the hybridization of the xenon trioxide.
- Therefore $s{{p}^{3}}+s$ type of overlapping is not present in xenon trioxide.
So, the correct option is D.
Note:
Xenon belongs to inert gases. Generally inert gases won’t react with other molecules. But in extreme conditions they react and form few derivatives as the products. Oxides of xenon belong to that category. The structure of xenon trioxide is trigonal pyramidal. In the structure of xenon trioxide there are three sigma bonds and three pi bonds. The oxidation state of xenon in xenon trioxide is ‘+6’.
Complete answer:
- In the question it is asked to find which overlapping among the given options is not present in the xenon trioxide.
- Xenon belongs to group VIII A in the periodic table.
- Oxygen belongs to group VI A in the periodic table.
- The orbital overlapping and hybridization of the oxygen atoms with the xenon atom is as follows.

- From the above picture we can say that $s{{p}^{3}}+{{p}_{x}}$ , $s{{p}^{3}}+{{p}_{y}}$ and $s{{p}^{3}}+{{p}_{z}}$ are involved in the hybridization of xenon trioxide.
- Coming to option C, ${{d}_{xz}}+{{p}_{x}}$ is also involved in the hybridization process of xenon trioxide.
- We cannot see any $s{{p}^{3}}+s$ type of overlapping in the hybridization of the xenon trioxide.
- Therefore $s{{p}^{3}}+s$ type of overlapping is not present in xenon trioxide.
So, the correct option is D.
Note:
Xenon belongs to inert gases. Generally inert gases won’t react with other molecules. But in extreme conditions they react and form few derivatives as the products. Oxides of xenon belong to that category. The structure of xenon trioxide is trigonal pyramidal. In the structure of xenon trioxide there are three sigma bonds and three pi bonds. The oxidation state of xenon in xenon trioxide is ‘+6’.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




