
Which of the following molecules will form a linear polymeric structure due to hydrogen bonding?
A. \[{\text{HCl}}\]
B. \[{\text{HF}}\]
C. \[{{\text{H}}_{\text{2}}}{\text{O}}\]
D. \[{\text{N}}{{\text{H}}_{\text{3}}}\]
Answer
569.7k+ views
Hint: Only molecules in which the H atom is bonded to either O, N, or F show hydrogen bonding. Draw the structures of molecules that show hydrogen bonding and determine which molecule form a linear polymeric structure due to hydrogen bonding
Complete answer:
- Intermolecular force is the force of attraction that holds the molecule together. There are various types of intermolecular forces like Van der Waals force, hydrogen bond, covalent attraction, ionic interaction, etc. The type of intermolecular forces depends on the type of molecules.
- Hydrogen bonding is the electrostatic force of attraction that binds the H-atom of one molecule with the electronegative atom of another molecule of the same substance.
- The electronegative atoms that show hydrogen bonding is O, N, or F, So molecules in which the H atom is bonded to either O, N, or F show polymerization due to hydrogen bonding.
- In the case of \[{\text{HCl}}\] we can see that hydrogen is bonded to chlorine atoms so it will not show hydrogen bonding.
All other molecules are given in options that are \[{\text{HF}}\], \[{{\text{H}}_{\text{2}}}{\text{O}}\] and \[{\text{N}}{{\text{H}}_{\text{3}}}\] shows the hydrogen bonding as in these molecules hydrogen atom is bonded to electronegative atoms O, N and F.
Now we will determine which molecule will form a linear polymeric structure due to hydrogen bonding as follows:
\[{\text{HF}}\]
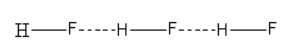
Here we can see that \[{\text{HF}}\] show a linear polymeric structure due to hydrogen bonding.
\[{{\text{H}}_{\text{2}}}{\text{O}}\]
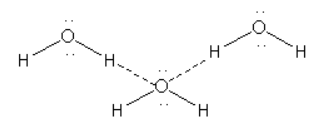
Here we can see that though \[{{\text{H}}_{\text{2}}}{\text{O}}\] show polymerisation due to hydrogen bonding it is not linear polymerisation.
\[{\text{N}}{{\text{H}}_{\text{3}}}\]
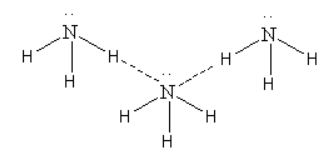
Here we can see that though \[{\text{N}}{{\text{H}}_{\text{3}}}\] show polymerisation due to hydrogen bonding it is not linear polymerisation.
Thus, the correct option is (B) \[{\text{HF}}\].
Note: When a hydrogen atom is a sandwich between two highly electronegative elements (F, O, N) it shows a unique property to form a bridge between them and it is known as hydrogen bonding. Only linear molecules show liner polymerisation due to hydrogen bonding
Complete answer:
- Intermolecular force is the force of attraction that holds the molecule together. There are various types of intermolecular forces like Van der Waals force, hydrogen bond, covalent attraction, ionic interaction, etc. The type of intermolecular forces depends on the type of molecules.
- Hydrogen bonding is the electrostatic force of attraction that binds the H-atom of one molecule with the electronegative atom of another molecule of the same substance.
- The electronegative atoms that show hydrogen bonding is O, N, or F, So molecules in which the H atom is bonded to either O, N, or F show polymerization due to hydrogen bonding.
- In the case of \[{\text{HCl}}\] we can see that hydrogen is bonded to chlorine atoms so it will not show hydrogen bonding.
All other molecules are given in options that are \[{\text{HF}}\], \[{{\text{H}}_{\text{2}}}{\text{O}}\] and \[{\text{N}}{{\text{H}}_{\text{3}}}\] shows the hydrogen bonding as in these molecules hydrogen atom is bonded to electronegative atoms O, N and F.
Now we will determine which molecule will form a linear polymeric structure due to hydrogen bonding as follows:
\[{\text{HF}}\]
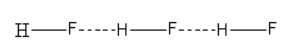
Here we can see that \[{\text{HF}}\] show a linear polymeric structure due to hydrogen bonding.
\[{{\text{H}}_{\text{2}}}{\text{O}}\]

Here we can see that though \[{{\text{H}}_{\text{2}}}{\text{O}}\] show polymerisation due to hydrogen bonding it is not linear polymerisation.
\[{\text{N}}{{\text{H}}_{\text{3}}}\]

Here we can see that though \[{\text{N}}{{\text{H}}_{\text{3}}}\] show polymerisation due to hydrogen bonding it is not linear polymerisation.
Thus, the correct option is (B) \[{\text{HF}}\].
Note: When a hydrogen atom is a sandwich between two highly electronegative elements (F, O, N) it shows a unique property to form a bridge between them and it is known as hydrogen bonding. Only linear molecules show liner polymerisation due to hydrogen bonding
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




