
Which of the following molecule(s) is/are chiral?
This question has multiple correct options.
A.
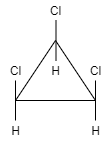
B.
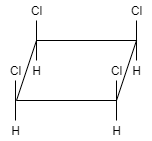
C.
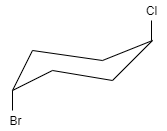
D.





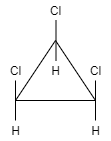
Answer
569.4k+ views
Hint: The compounds which cannot be superimposed on their mirror image by the combination of rotation are termed as chiral compounds. They exist in two isomers which show mirror images of each other .
Complete step by step answer:
A chiral molecule consists of a chiral center. When the center coincides with another atom, then that substance is having point chirality. A compound is termed as a chiral compound when it does not have a plane of symmetry and center of symmetry. If the compound does not have any of these then it is said to be chiral.
The Plane of symmetry is the plane which divides a molecule into 2 halves that shows mirror images of each other.
Center of symmetry is the point from where if we move towards two equivalent groups ,it shows equal distance.
If a compound shows a plane of symmetry and center of symmetry, then it is an achiral compound. And if a compound is showing only either plane of symmetry or center of symmetry, then also it is an achiral compound.
Now let us discuss these options given above, one by one.
A.
This molecule shows a plane of symmetry but it does not show a center of symmetry.
It is an achiral compound and therefore this option is incorrect.
B.
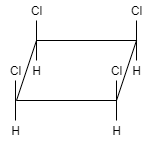
This molecule shows a plane of symmetry as well as center of symmetry. Therefore, this compound is achiral. So, this option is also incorrect.
C.
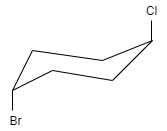
This molecule shows neither plane of symmetry nor center of symmetry. Therefore, this compound is chiral and hence this option is correct.
D.

This molecule shows neither plane of symmetry nor center of symmetry. Therefore, this compound is chiral and hence this option is correct.
So, the correct answer is Option C,D.
Note: Chiral compounds do not show superimposable mirror images, whereas achiral compounds show superimposable mirror images of each other.
In the conclusion, when a compound that shows both plane of symmetry and center of symmetry or, it shows either plane of symmetry or center of symmetry, that compound is known as achiral compound.
Complete step by step answer:
A chiral molecule consists of a chiral center. When the center coincides with another atom, then that substance is having point chirality. A compound is termed as a chiral compound when it does not have a plane of symmetry and center of symmetry. If the compound does not have any of these then it is said to be chiral.
The Plane of symmetry is the plane which divides a molecule into 2 halves that shows mirror images of each other.
Center of symmetry is the point from where if we move towards two equivalent groups ,it shows equal distance.
If a compound shows a plane of symmetry and center of symmetry, then it is an achiral compound. And if a compound is showing only either plane of symmetry or center of symmetry, then also it is an achiral compound.
Now let us discuss these options given above, one by one.
A.

This molecule shows a plane of symmetry but it does not show a center of symmetry.
It is an achiral compound and therefore this option is incorrect.
B.

This molecule shows a plane of symmetry as well as center of symmetry. Therefore, this compound is achiral. So, this option is also incorrect.
C.

This molecule shows neither plane of symmetry nor center of symmetry. Therefore, this compound is chiral and hence this option is correct.
D.

This molecule shows neither plane of symmetry nor center of symmetry. Therefore, this compound is chiral and hence this option is correct.
So, the correct answer is Option C,D.
Note: Chiral compounds do not show superimposable mirror images, whereas achiral compounds show superimposable mirror images of each other.
In the conclusion, when a compound that shows both plane of symmetry and center of symmetry or, it shows either plane of symmetry or center of symmetry, that compound is known as achiral compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




