
Which of the following molecules has a see-saw geometry?
A.$I_{3}^{-}$
B.$ICl_{2}^{-}$
C.$ClF_{3}^{-}$
D.$I{{O}_{2}}F_{2}^{-}$
Answer
566.7k+ views
Hint: To find the geometry and shape of any molecule use VSEPR theory. Shape of a molecule can also be determined through its lewis structure . In lewis structure there are lone pairs and bond pairs that help in finding the hybridization and shape of a molecule.
Complete answer:
From your chemistry lessons you are aware of how to draw the lewis structure of any molecule. Through this you can find the hybridization and the shape of any molecule using the VSEPR theory. There are more ways to find the shape .
-Here we are going to use Lewis structure concept to find the answer
-We are going to draw the lewis structure of one of the given molecule in the question ,
(1)$I{{O}_{2}}F_{2}^{-}$
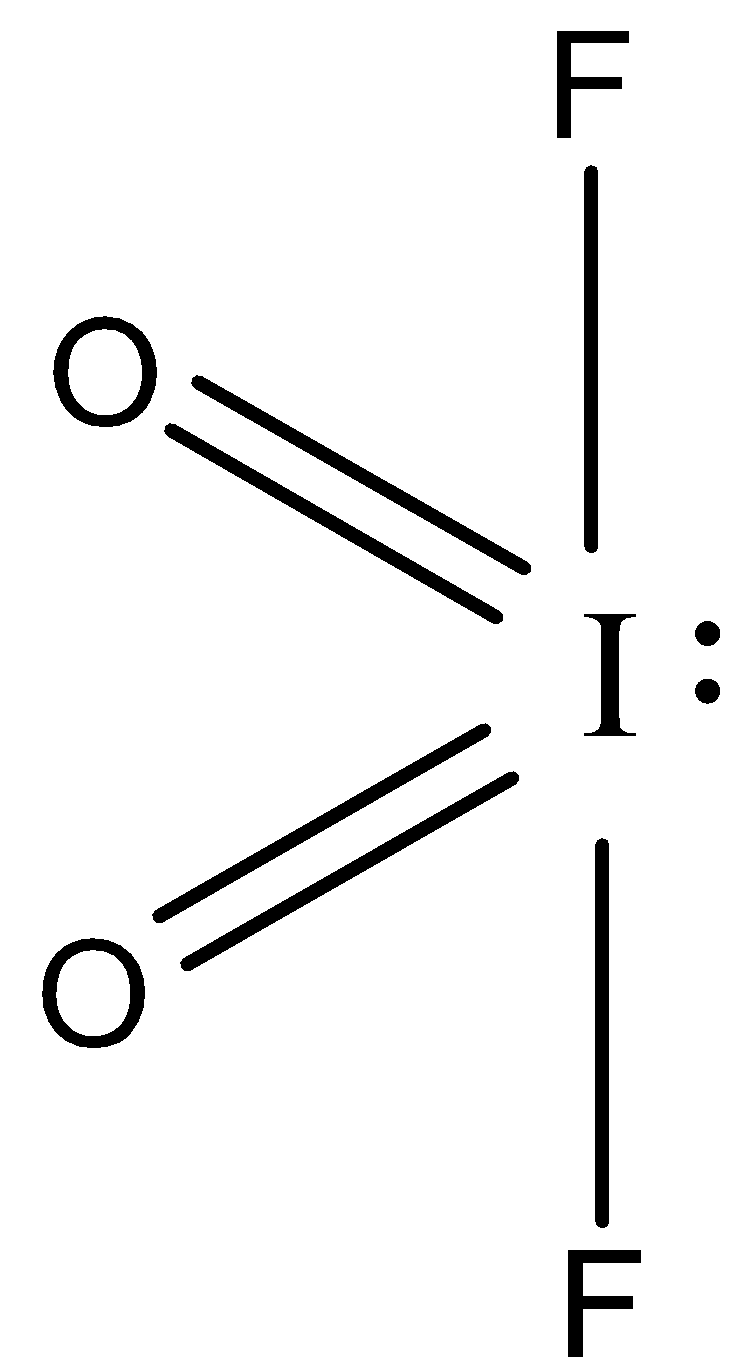
-So, the no of valence electrons of iodine is 7.
- Here iodine shares one of its electrons with fluorine forming a single bond and two of its electrons with oxygen to form a double bond and one lone pair.
Now we will find the hybridisation,
-Firstly the formula to find the hybridization is,
\[\dfrac{1}{2}\left( V+H-C+A \right)\]
Where, V= Valence electrons in the central atom
H= no of surrounding monovalent atoms
C= Cationic Charge
A= Anionic Charge
Now, for the first case the value of V=7 and H=2(here we will take h as 2 because oxygen is a divalent atom and not monovalent but fluorine is a monovalent atom)and A=1, by putting all the values you will get
\[\dfrac{1}{2}\left( 7+2+1 \right)\,=\,\dfrac{10}{2}\,=5\]
Thus the hybridization will be $s{{p}_{3}}d$, hence the shape will be trigonal pyramidal but due to the presence of lone pair one bond will not be present and thus it will show see-saw geometry as you can see from the lewis structure.
-In this way you can find the geometry.
Thus, the correct option will be (D).
Note: Hybridization should be written correctly. Lone pair and bond pair should be checked so you can draw the correct geometry. Find the central atom of the molecule and arrange the no of valence electrons and then put the surrounding atoms around the central atom.
Complete answer:
From your chemistry lessons you are aware of how to draw the lewis structure of any molecule. Through this you can find the hybridization and the shape of any molecule using the VSEPR theory. There are more ways to find the shape .
-Here we are going to use Lewis structure concept to find the answer
-We are going to draw the lewis structure of one of the given molecule in the question ,
(1)$I{{O}_{2}}F_{2}^{-}$

-So, the no of valence electrons of iodine is 7.
- Here iodine shares one of its electrons with fluorine forming a single bond and two of its electrons with oxygen to form a double bond and one lone pair.
Now we will find the hybridisation,
-Firstly the formula to find the hybridization is,
\[\dfrac{1}{2}\left( V+H-C+A \right)\]
Where, V= Valence electrons in the central atom
H= no of surrounding monovalent atoms
C= Cationic Charge
A= Anionic Charge
Now, for the first case the value of V=7 and H=2(here we will take h as 2 because oxygen is a divalent atom and not monovalent but fluorine is a monovalent atom)and A=1, by putting all the values you will get
\[\dfrac{1}{2}\left( 7+2+1 \right)\,=\,\dfrac{10}{2}\,=5\]
Thus the hybridization will be $s{{p}_{3}}d$, hence the shape will be trigonal pyramidal but due to the presence of lone pair one bond will not be present and thus it will show see-saw geometry as you can see from the lewis structure.
-In this way you can find the geometry.
Thus, the correct option will be (D).
Note: Hybridization should be written correctly. Lone pair and bond pair should be checked so you can draw the correct geometry. Find the central atom of the molecule and arrange the no of valence electrons and then put the surrounding atoms around the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




