
Which of the following molecules are chiral?
(I)
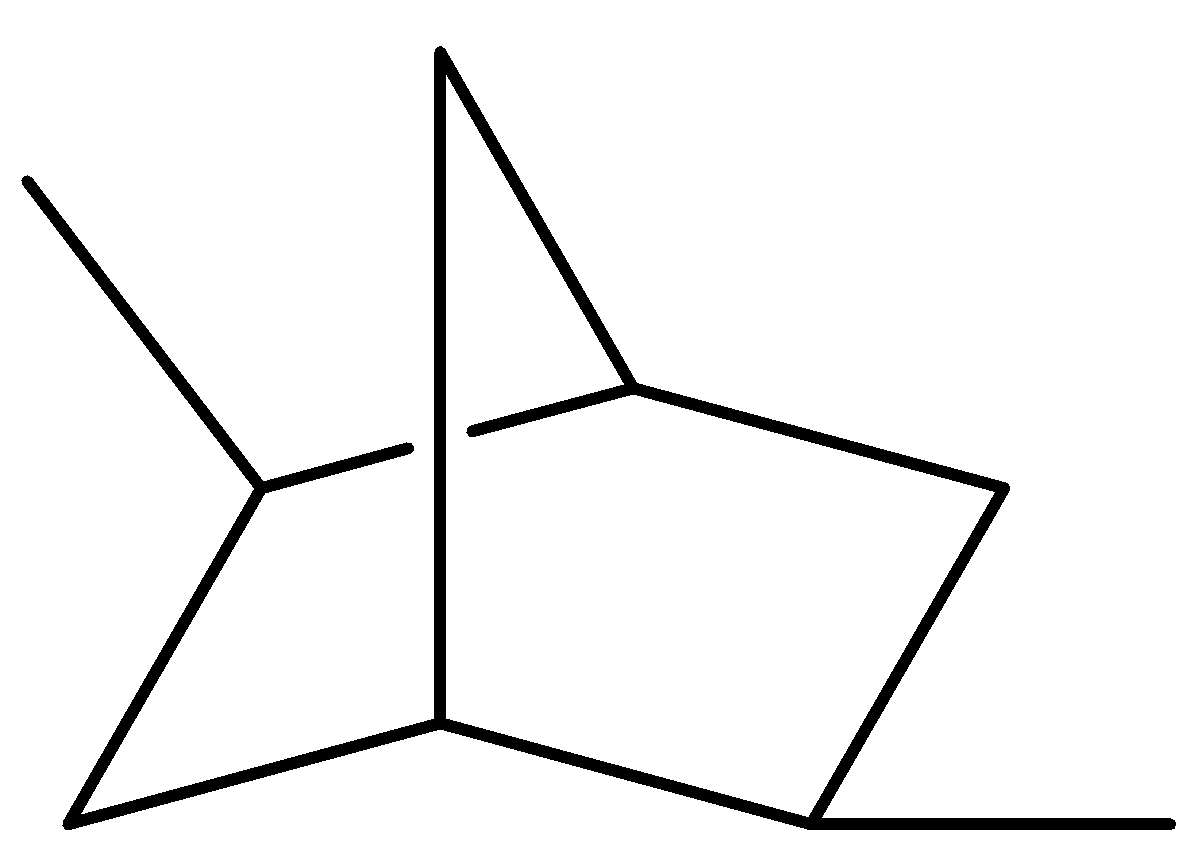
(II)

(III)
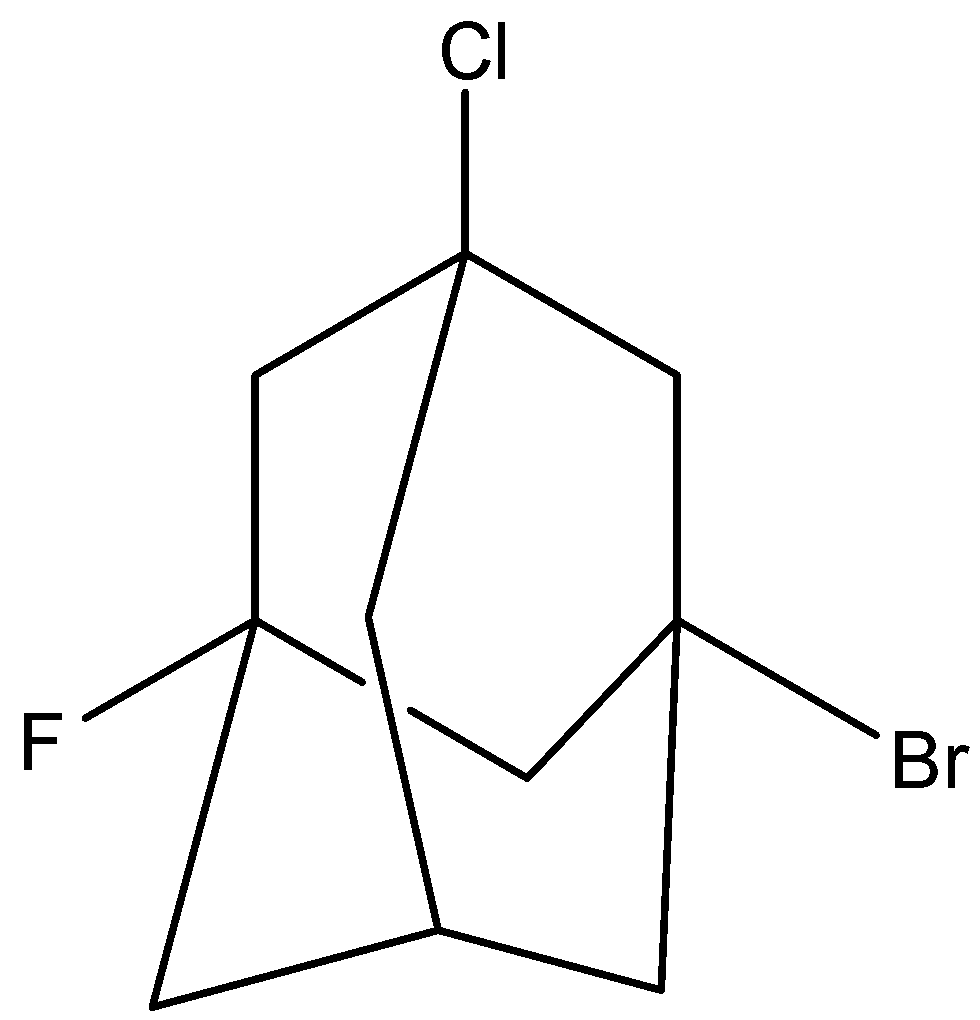
(IV)
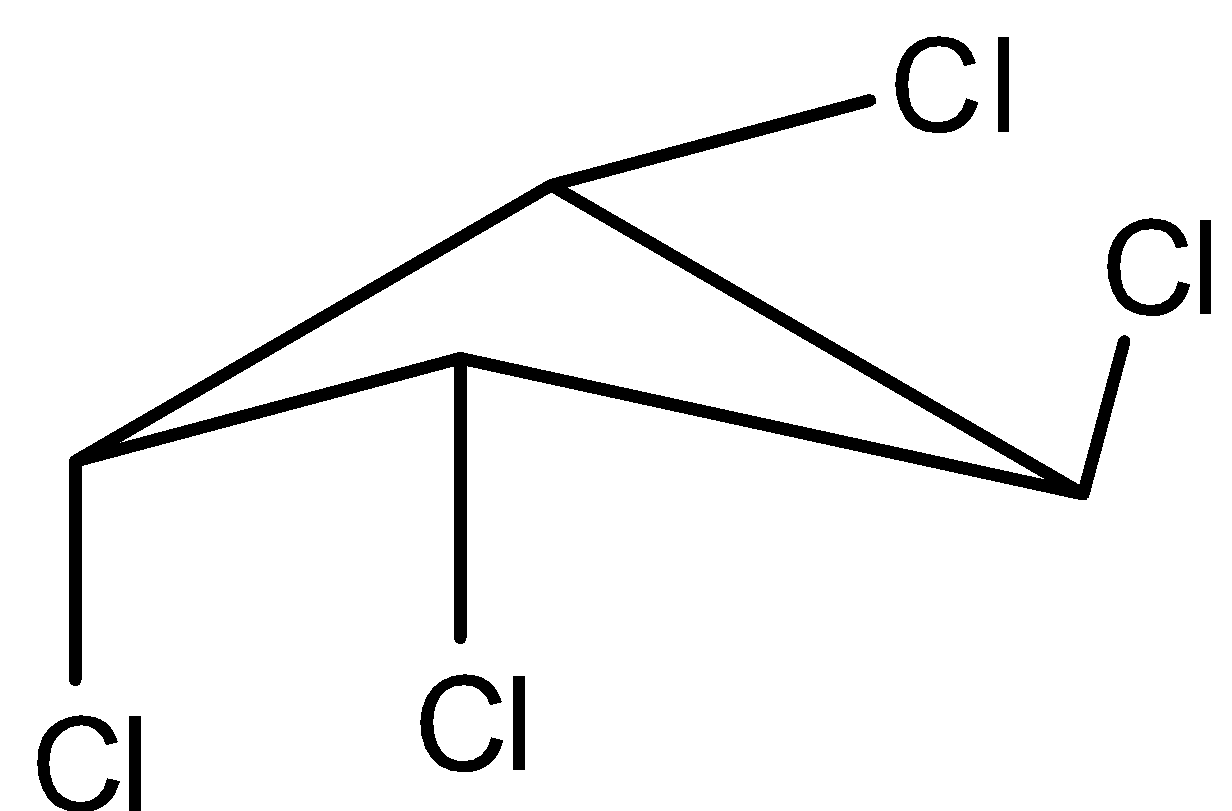
A.I,III
B.II,III,IV
C.II, IV
D. All of these




Answer
565.5k+ views
Hint: We know that a molecule is called chiral that has non-superimposable mirror image. For example, our hands are an example of chiral objects. Generally a molecule is called chiral if it does not have a plane of symmetry.
Complete step by step answer:
Let’s understand the plane of symmetry in detail. A plane of symmetry is an imaginary plane that divides the molecules equally into two halves which are mirror images of each other. We know a molecule is called chiral if it has non-superimposable mirror image. So, if a molecule has a plane of symmetry then the molecule is not chiral. We have to use this principle to identify whether the given molecules are chiral or not.
1 molecule is,
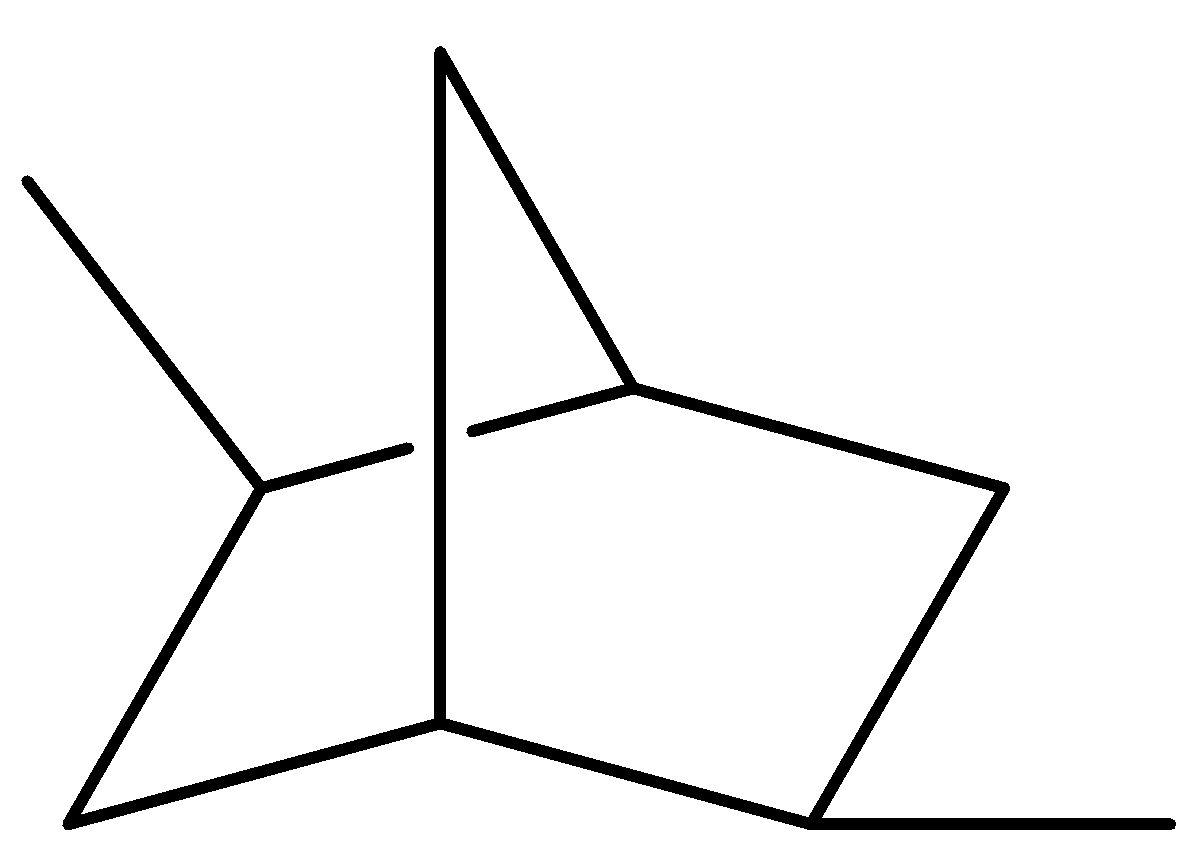
Here, no plane of symmetry present as bisecting the molecule does not result in a superimposable image. And we know if a molecule does not have a plane of symmetry, the molecule is chiral. So, the molecule is chiral.
2 molecule is

If we convert the above Newman Projection to the Fisher projection we get the structure,
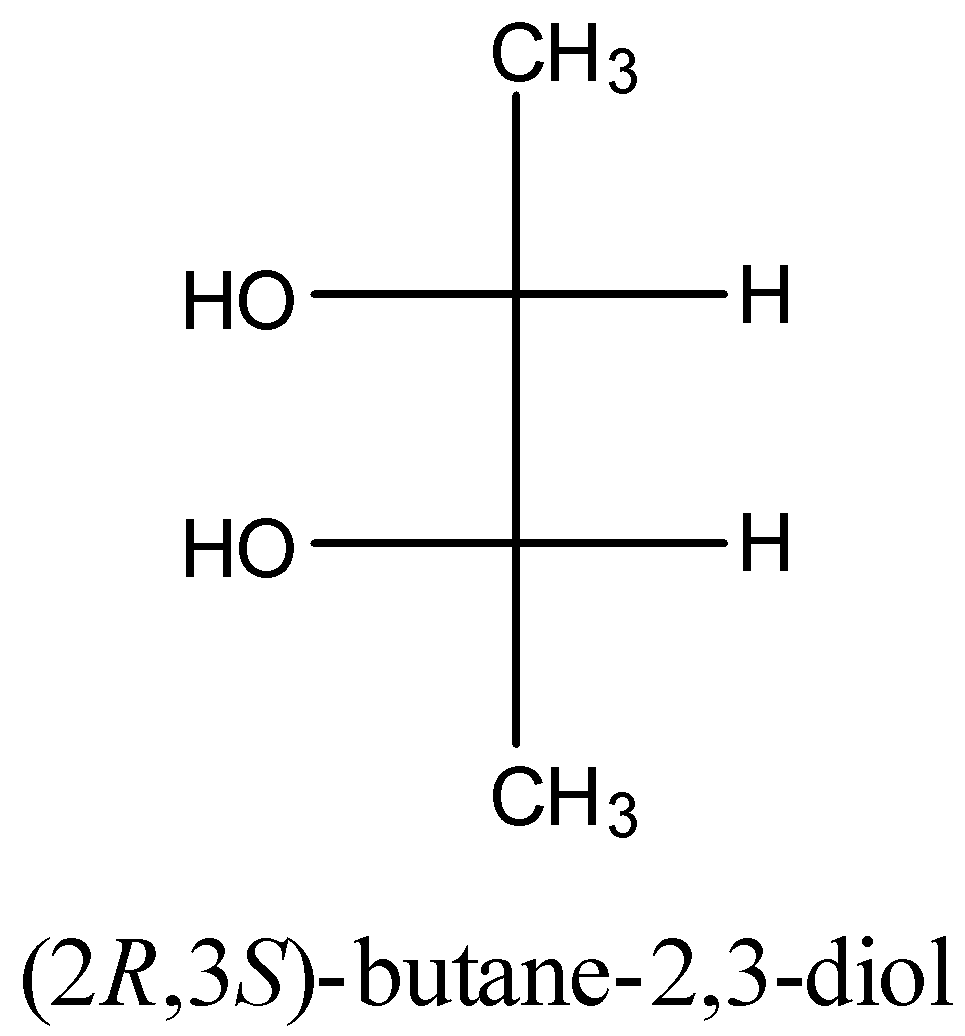
We clearly see that the above molecule has a plane of symmetry, so the molecule is achiral.
3 molecule is,
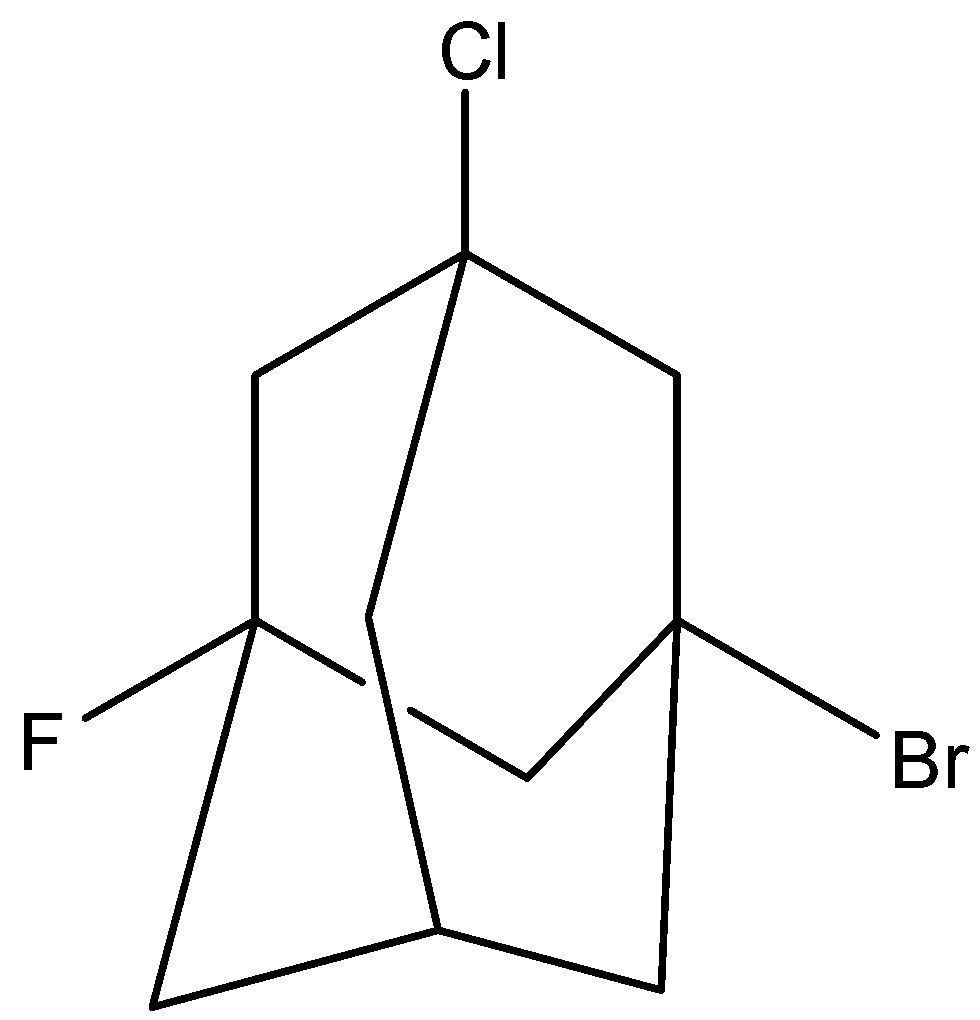
This molecule is chiral as no plane of symmetry present.
Fourth molecule is,
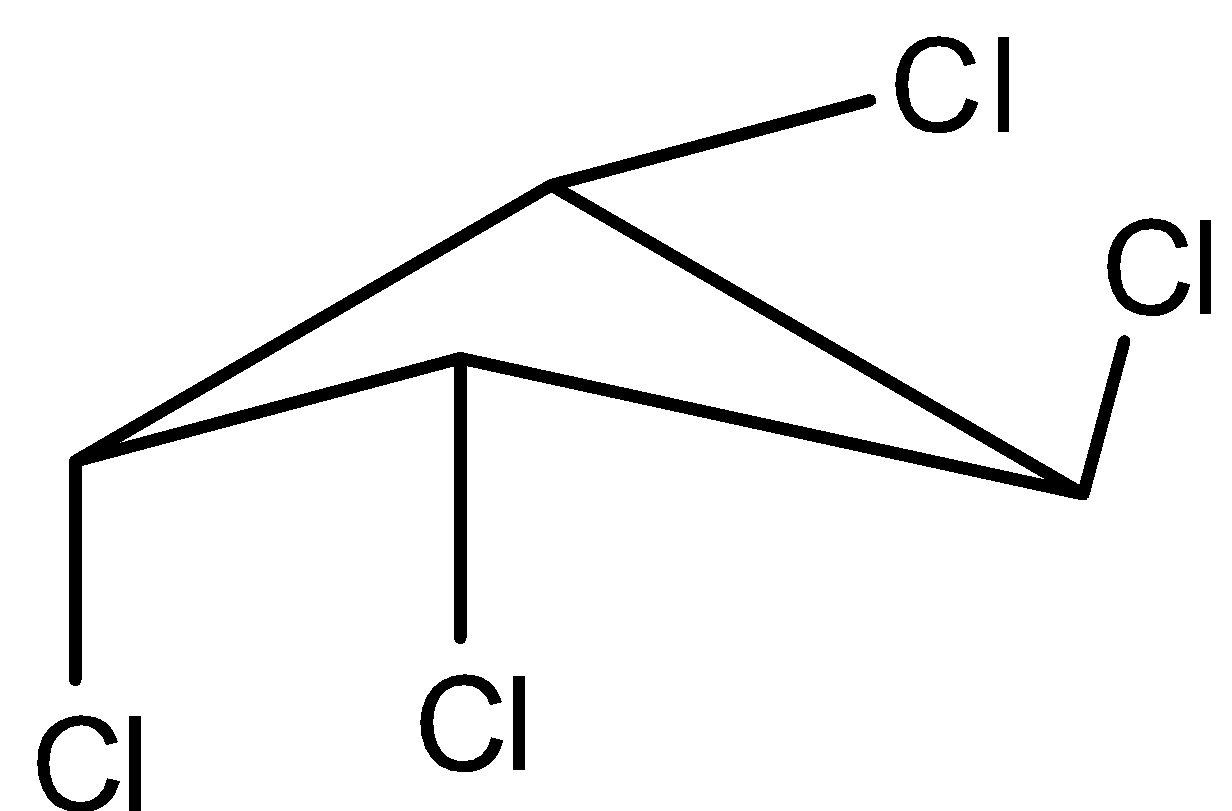
The molecule has a plane of symmetry. So, it is achiral.
Hence, option A is the correct answer.
Note: Always remember that molecules that can exist as enantiomers are called chiral and they are superimposable on their mirror images. Chirality is the sufficient and necessary condition for the existence of isomers. Molecules that are superimposable on their mirror images are said to be achiral.
Complete step by step answer:
Let’s understand the plane of symmetry in detail. A plane of symmetry is an imaginary plane that divides the molecules equally into two halves which are mirror images of each other. We know a molecule is called chiral if it has non-superimposable mirror image. So, if a molecule has a plane of symmetry then the molecule is not chiral. We have to use this principle to identify whether the given molecules are chiral or not.
1 molecule is,

Here, no plane of symmetry present as bisecting the molecule does not result in a superimposable image. And we know if a molecule does not have a plane of symmetry, the molecule is chiral. So, the molecule is chiral.
2 molecule is

If we convert the above Newman Projection to the Fisher projection we get the structure,

We clearly see that the above molecule has a plane of symmetry, so the molecule is achiral.
3 molecule is,

This molecule is chiral as no plane of symmetry present.
Fourth molecule is,

The molecule has a plane of symmetry. So, it is achiral.
Hence, option A is the correct answer.
Note: Always remember that molecules that can exist as enantiomers are called chiral and they are superimposable on their mirror images. Chirality is the sufficient and necessary condition for the existence of isomers. Molecules that are superimposable on their mirror images are said to be achiral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




