
Which of the following metals is the most reactive?
(A) Fe
(B) Zn
(C) Ca
(D) Al
Answer
595.2k+ views
Hint: The more reactive metals always displace less reactive metals from the latter’s compounds to form their own. In other ways we can also relate their reactivity with their oxidation potential that they both are directly proportional to each other.
Complete step by step solution:
To be able to answer this question effectively, we need to first understand what the reactivity series of metals really is and what is its order. So, let us first begin by trying to understand this concept.
The metal reactivity series involves the placement of metals, as its name suggests, in order of reactivity from most reactive to least reactive. It’s also a useful tool in predicting the products of simple displacement reactions involving two different metals, as well as providing an insight into why different metals are extracted from their ores in different manners.
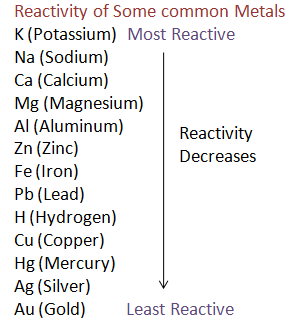
Group 1 metals, the most reactive metals in the periodic table, lead the rankings. They’re closely followed by the marginally less reactive group two metals. The metals designated as the transition metals in the periodic table are much less reactive, and metals such as gold and platinum prop up the bottom of the series, exhibiting little in the way of chemical reaction with any everyday reagents.
- Another way is to compare their oxidation potentials as oxidation potential shows the ability of metals to get oxidised. If metal will react, they will only get oxidised. So, we can say that both oxidation potential and reactivity of metals are directly proportional to each other. Oxidation potential of Fe, Zn , Ca and Al is 0.44, 0.76, 1.66 and 2.87 volts respectively.
Therefore, by this reactivity order of metals, we can safely conclude that the answer to this question is (C) Ca.
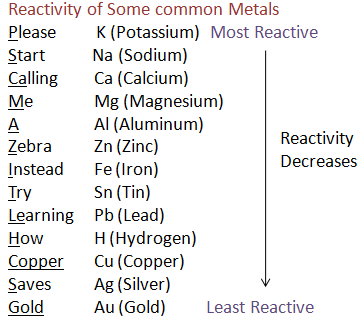
Note: An effective mnemonic device to help remember the reactivity order of metals is:
Please Start Calling Me A Careless Zebra, Instead Try Learning How Copper Saves Gold.
Complete step by step solution:
To be able to answer this question effectively, we need to first understand what the reactivity series of metals really is and what is its order. So, let us first begin by trying to understand this concept.
The metal reactivity series involves the placement of metals, as its name suggests, in order of reactivity from most reactive to least reactive. It’s also a useful tool in predicting the products of simple displacement reactions involving two different metals, as well as providing an insight into why different metals are extracted from their ores in different manners.

Group 1 metals, the most reactive metals in the periodic table, lead the rankings. They’re closely followed by the marginally less reactive group two metals. The metals designated as the transition metals in the periodic table are much less reactive, and metals such as gold and platinum prop up the bottom of the series, exhibiting little in the way of chemical reaction with any everyday reagents.
- Another way is to compare their oxidation potentials as oxidation potential shows the ability of metals to get oxidised. If metal will react, they will only get oxidised. So, we can say that both oxidation potential and reactivity of metals are directly proportional to each other. Oxidation potential of Fe, Zn , Ca and Al is 0.44, 0.76, 1.66 and 2.87 volts respectively.
Therefore, by this reactivity order of metals, we can safely conclude that the answer to this question is (C) Ca.
Note: An effective mnemonic device to help remember the reactivity order of metals is:
Please Start Calling Me A Careless Zebra, Instead Try Learning How Copper Saves Gold.

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




