
Which of the following metals is the lightest in the periodic table of elements?
A) Lithium
B) Aluminium
C) Titanium
D) Magnesium
Answer
494.4k+ views
Hint: In a modern periodic table, the known elements are arranged in the horizontal rows (known as periods) and the vertical columns (known as groups) according to their chemical properties and atomic numbers. When we move from left to right in the periodic table, we will see that the atomic mass and atomic number of elements increase and they become heavier. So, we will see the atomic numbers and placement of the given elements in the periodic table to determine the lightest element among the given elements.
Complete answer:
We know that in a modern periodic table, the known elements are arranged in the horizontal rows (known as periods) and the vertical columns (known as groups). The elements are arranged according to their chemical properties and atomic numbers.
The greater the atomic mass/ atomic number, the heavier the element.
Now, we will look at the modern periodic table:
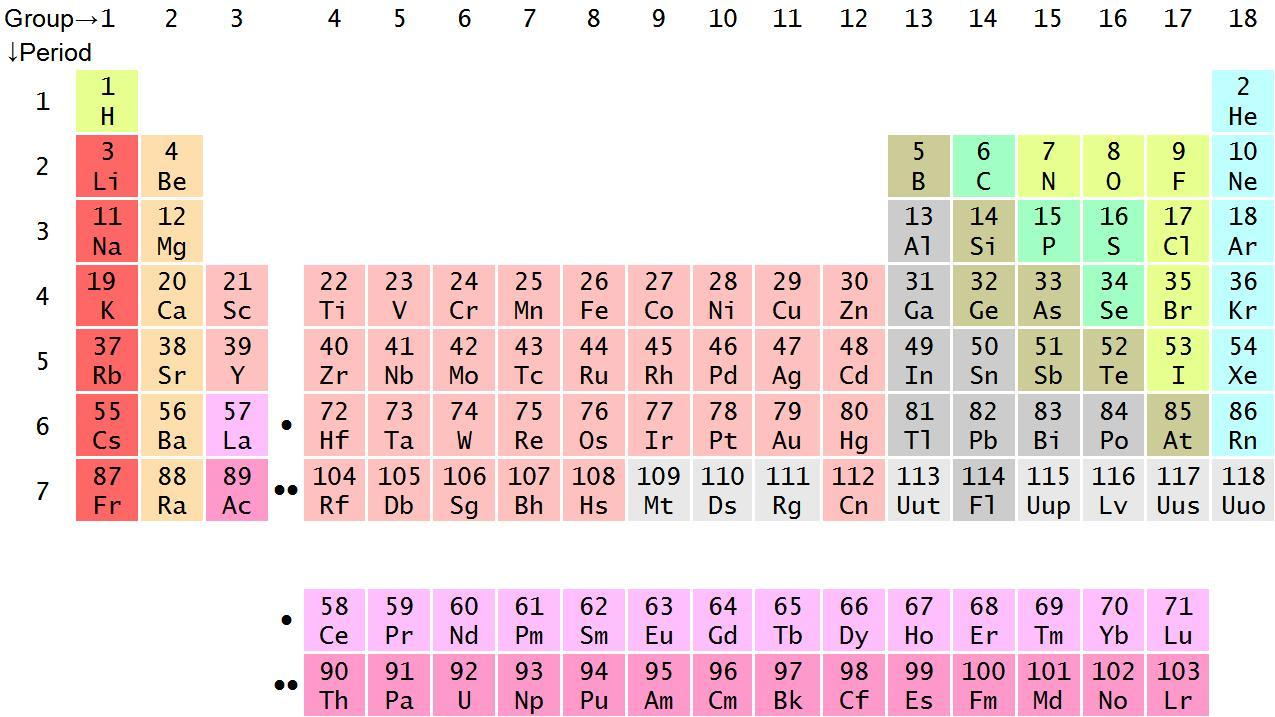
So, from the above periodic table, we can see that, the atomic number of Lithium (Li) is $3.$
Atomic number of Aluminium is $13.$
Atomic number of Titanium (Ti) is $22.$
Atomic number of Magnesium (Mg) is $12.$
Hence, we can see that the lightest metals from the above given metals is Lithium.
Therefore, Lithium is the lightest element in the periodic table. So, option (A) is correct.
Note:
Metals have certain characteristic physical and chemical properties such as they are ductile, malleable, lustrous, good conductors of electricity and heat. They also have high density and high melting points. Metals react with non-metals to form ionic compounds which have strong forces of attraction.
Complete answer:
We know that in a modern periodic table, the known elements are arranged in the horizontal rows (known as periods) and the vertical columns (known as groups). The elements are arranged according to their chemical properties and atomic numbers.
The greater the atomic mass/ atomic number, the heavier the element.
Now, we will look at the modern periodic table:

So, from the above periodic table, we can see that, the atomic number of Lithium (Li) is $3.$
Atomic number of Aluminium is $13.$
Atomic number of Titanium (Ti) is $22.$
Atomic number of Magnesium (Mg) is $12.$
Hence, we can see that the lightest metals from the above given metals is Lithium.
Therefore, Lithium is the lightest element in the periodic table. So, option (A) is correct.
Note:
Metals have certain characteristic physical and chemical properties such as they are ductile, malleable, lustrous, good conductors of electricity and heat. They also have high density and high melting points. Metals react with non-metals to form ionic compounds which have strong forces of attraction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




