
Which of the following leads to bonding?
A)
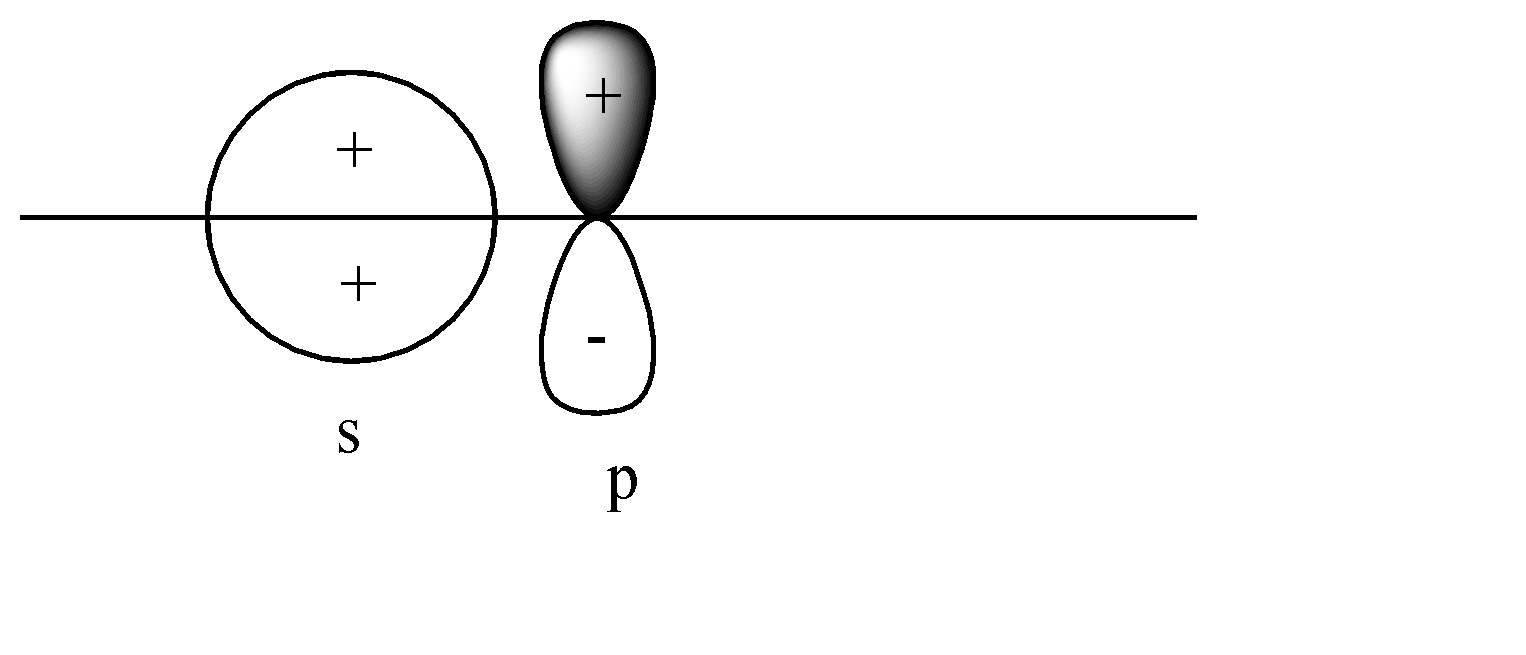
B)
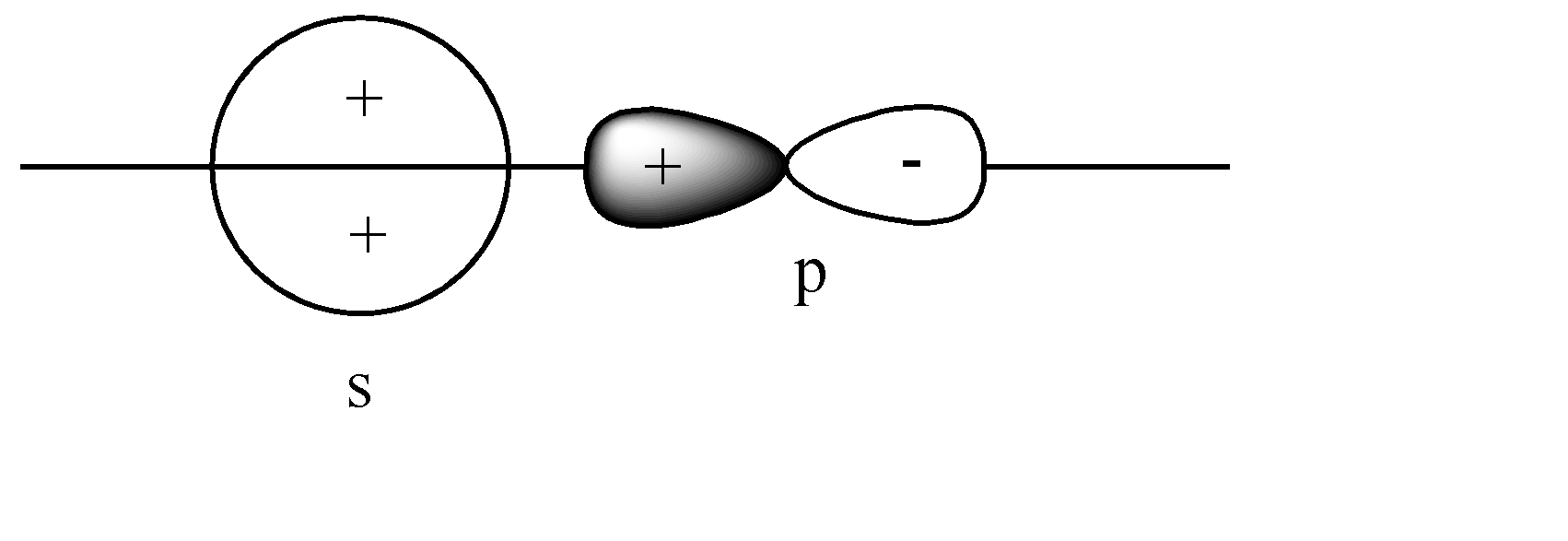
C)
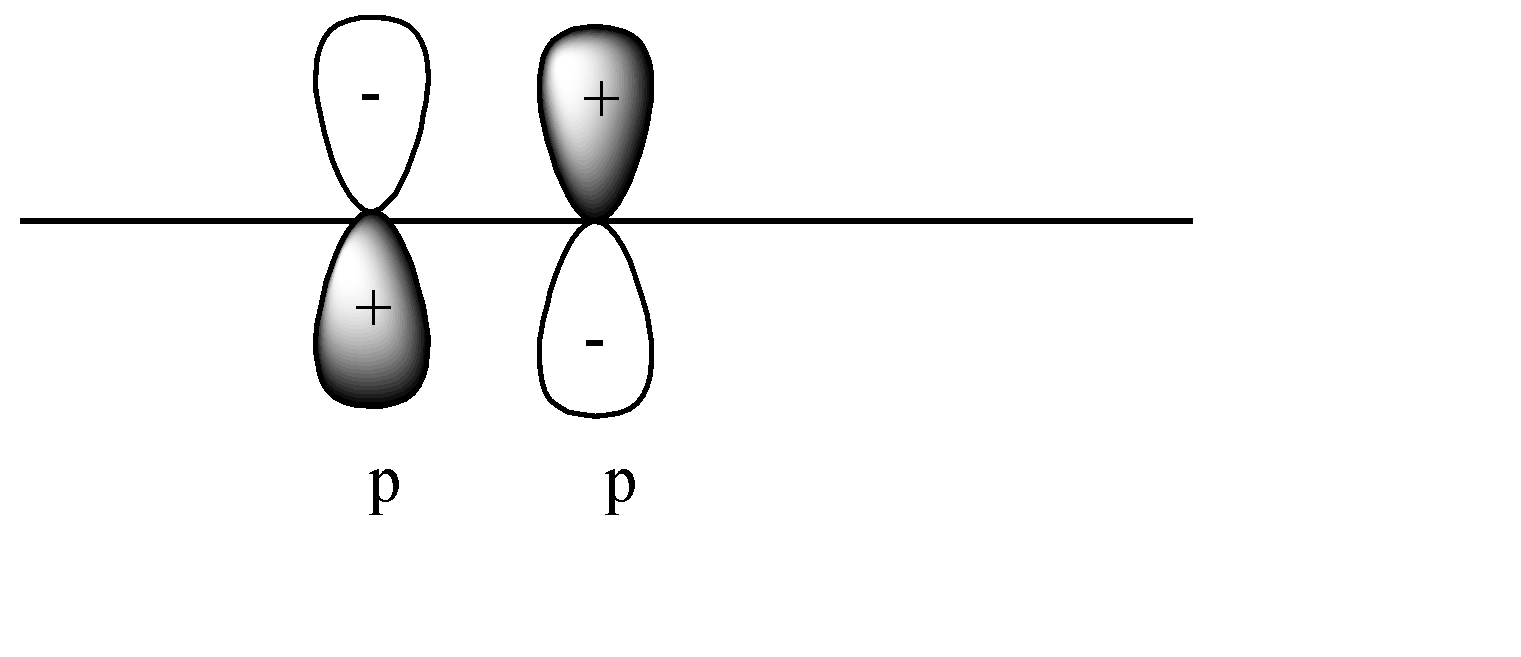
D)
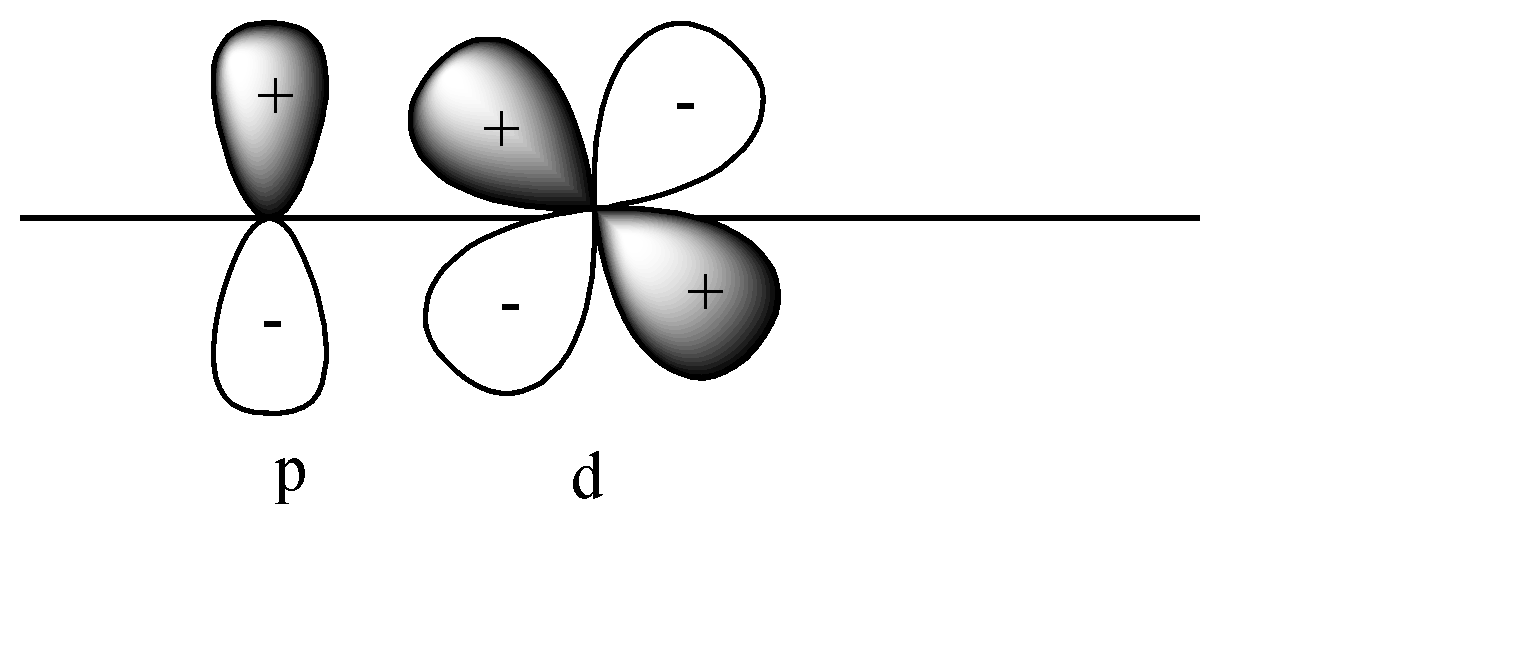




Answer
489.6k+ views
Hint: To answer this question, we have to recall the concept of sigma bond formation by mixing of orbitals. The + and – refers to the electron density dispersion across the orbitals. The formation of covalent bonds happens on collision of orbitals. Some lead to constructive interference and some destructive.
Complete answer:
According to the VSEPR theory there are two types of bonds that can be formed by collision of orbitals:
1. Sigma Bond
2. Pi Bond
The formation of sigma bonds occurs due to head-to-head collision of the orbitals that lie in the same plane or are coaxial/collinear. By definition, a sigma bond is formed by overlapping atomic orbitals that are in line with the internuclear axis. The s-orbital is spherical in shape and is said to be non-directional. P- orbitals have a dumbbell shape, and in order to form a sigma bond, p orbital must lie on the internuclear axis.
Let’s analyze the given options:
A)
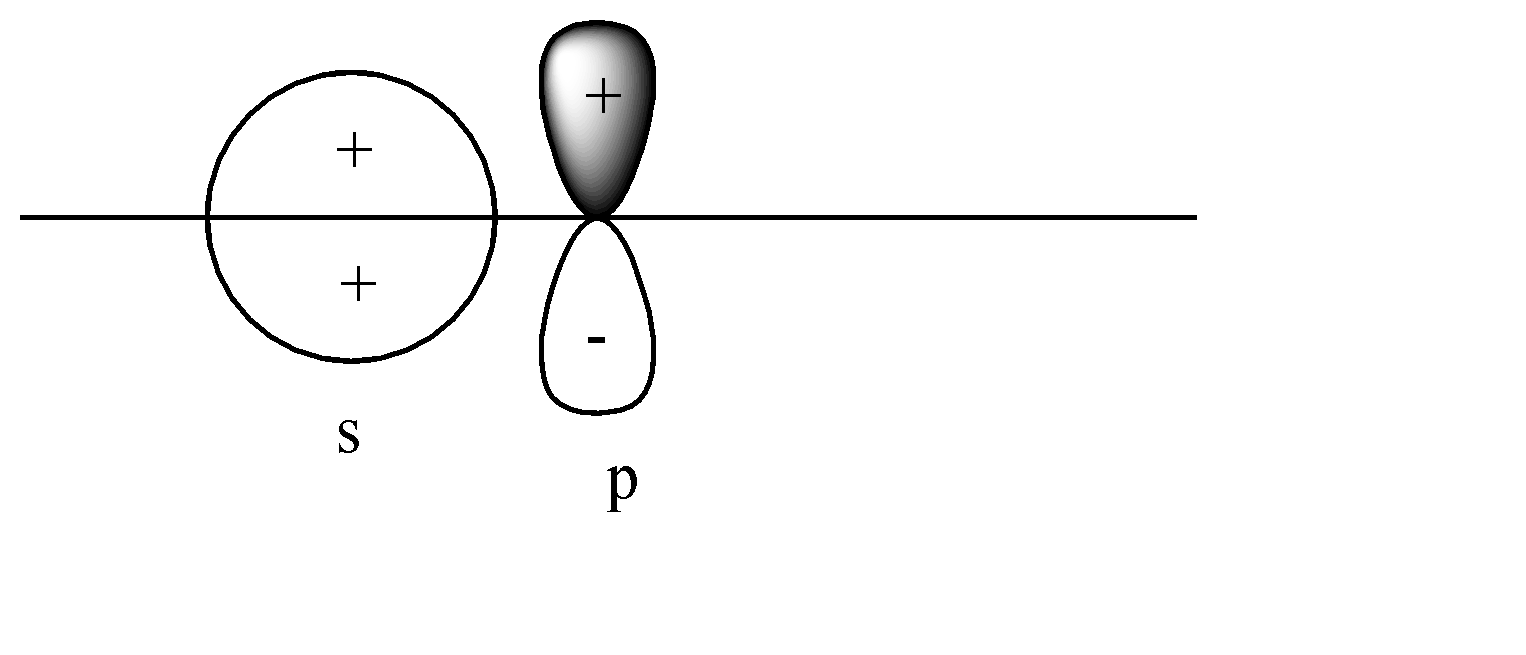
Here the s-orbital is present on the axis, but the p-orbital is perpendicular to the axis. Head-to-head collision is not possible; hence no formation of bond will take place.
B)
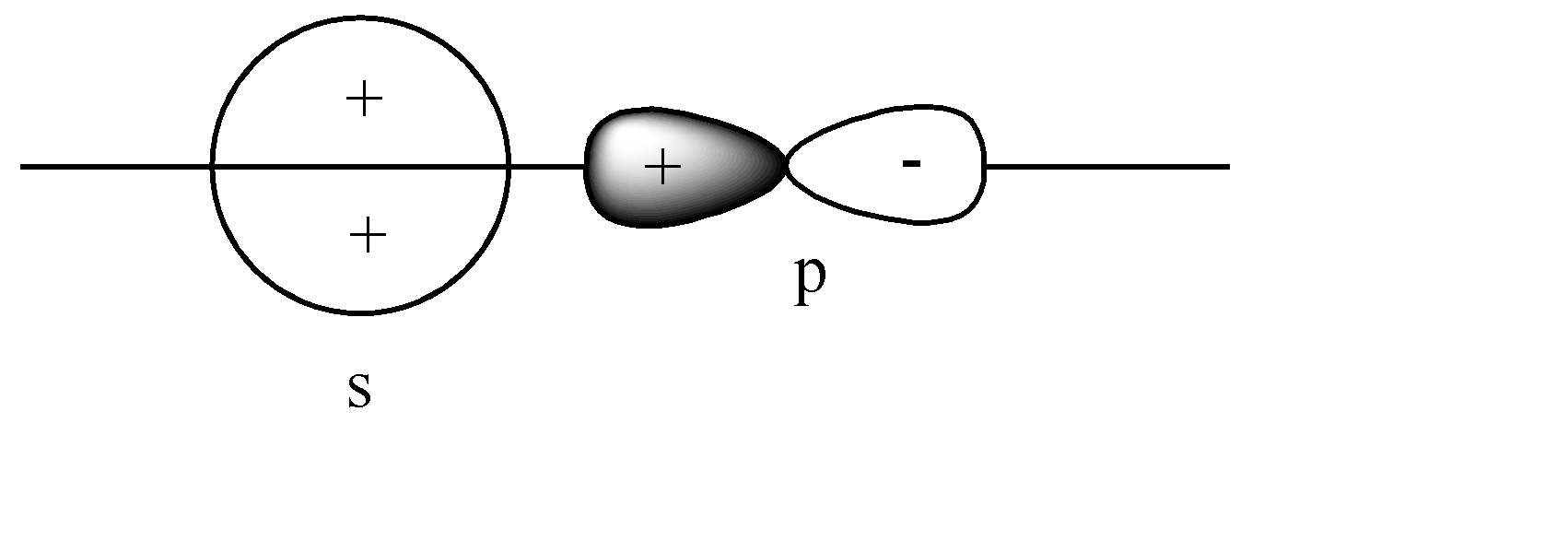
The s-orbital is present on the axis, and the p orbital is also parallel to the axis. Hence head-to-head collision is possible. Since the like charge i.e., ‘+ charge’ overlap, this leads to a constructive overlap, hence forming a Bonding sigma bond. This is the correct answer.
C)
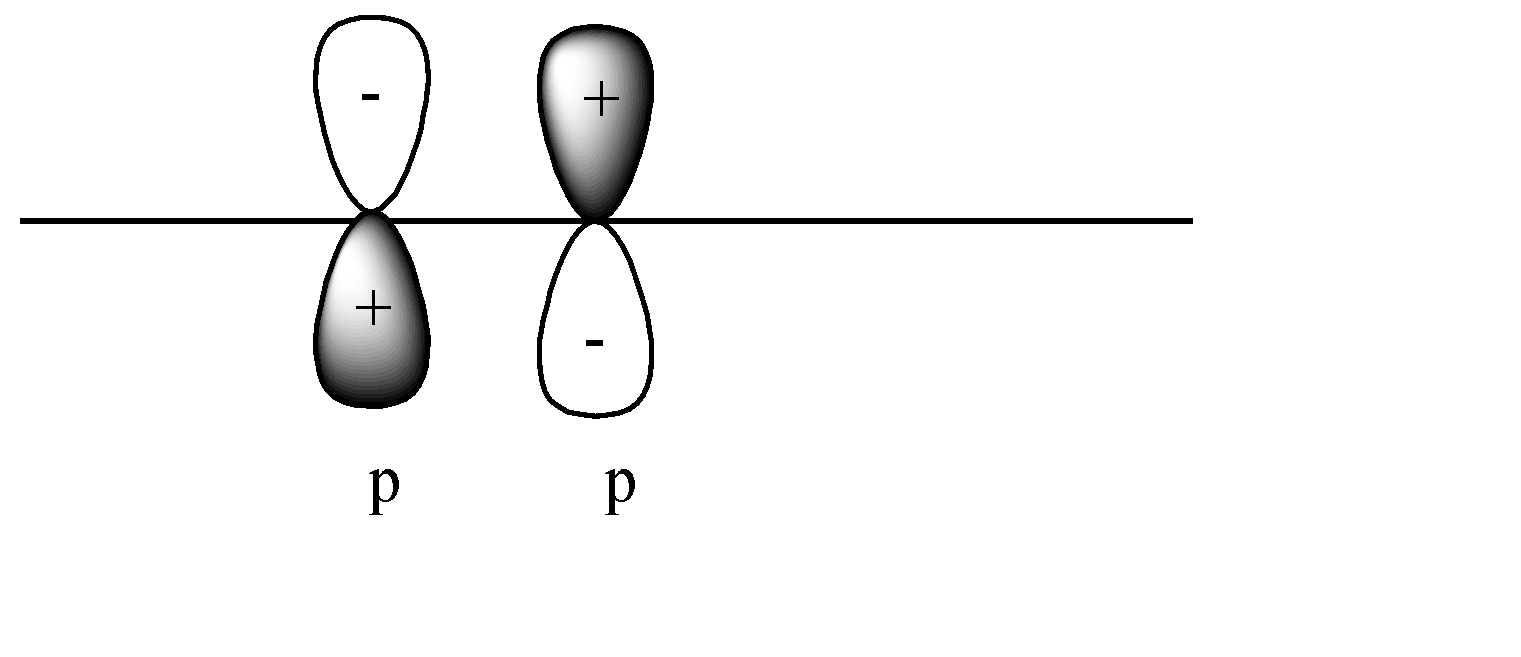
In this case a pi covalent bond will be formed by lateral overlap, or shoulder-to-shoulder overlap. In this case + orbital overlaps with – orbital, and vice versa. This leads to destructive overlap and formation of antibonding pi bonds.
D)
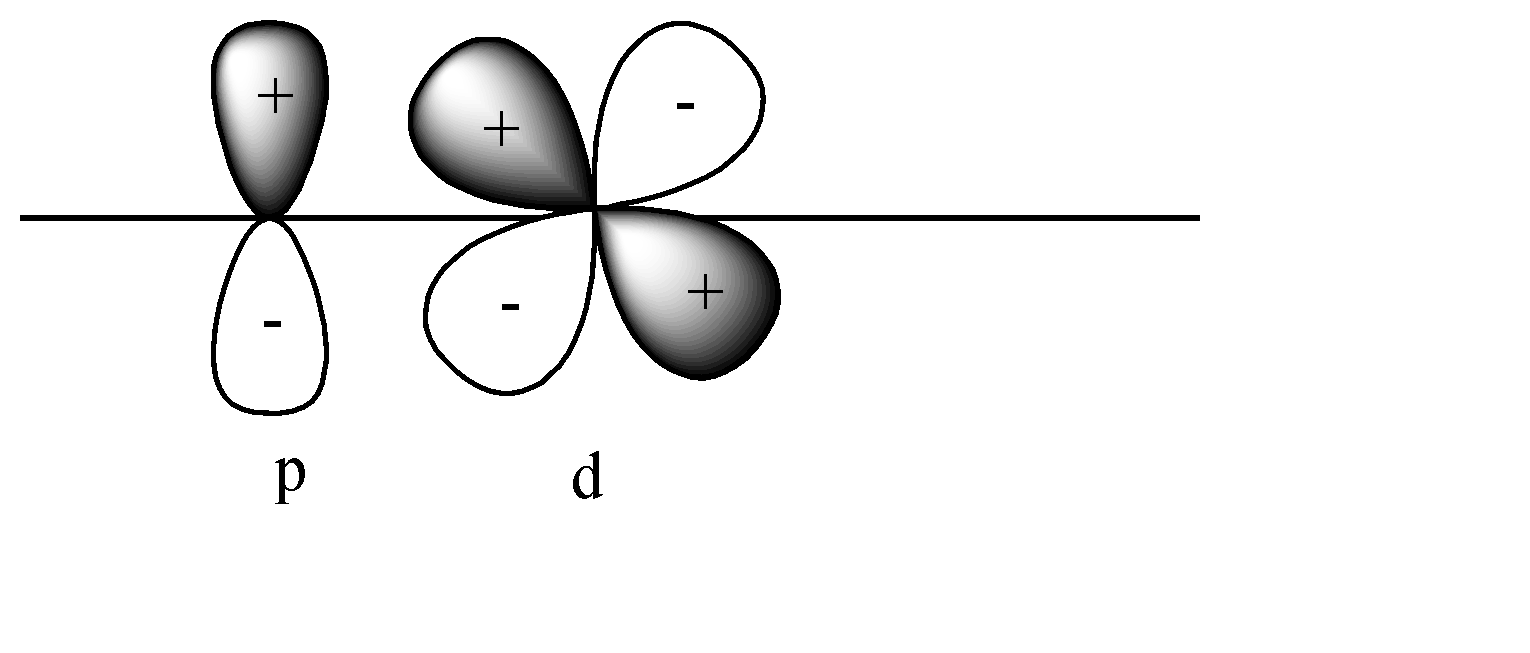
In this case also pi bonds will be formed as both p and d orbitals are perpendicular to the axis. In this case + orbital overlaps with – orbital, and vice versa. This leads to destructive overlap and formation of antibonding pi bonds.
Therefore, we can conclude that option (B) is the correct answer.
Note:
If $s$ orbital combines with a $p$ orbital perpendicular to the plane, no bond formation takes place and this forms a NBMO (non-bonding molecular orbital). Similarly with two p-orbitals, one in axis and one perpendicular to the axis, for example ${p_x}\& {p_y}$ orbitals on x-axis, will lead to a formation of NBMO.
Complete answer:
According to the VSEPR theory there are two types of bonds that can be formed by collision of orbitals:
1. Sigma Bond
2. Pi Bond
The formation of sigma bonds occurs due to head-to-head collision of the orbitals that lie in the same plane or are coaxial/collinear. By definition, a sigma bond is formed by overlapping atomic orbitals that are in line with the internuclear axis. The s-orbital is spherical in shape and is said to be non-directional. P- orbitals have a dumbbell shape, and in order to form a sigma bond, p orbital must lie on the internuclear axis.
Let’s analyze the given options:
A)

Here the s-orbital is present on the axis, but the p-orbital is perpendicular to the axis. Head-to-head collision is not possible; hence no formation of bond will take place.
B)

The s-orbital is present on the axis, and the p orbital is also parallel to the axis. Hence head-to-head collision is possible. Since the like charge i.e., ‘+ charge’ overlap, this leads to a constructive overlap, hence forming a Bonding sigma bond. This is the correct answer.
C)

In this case a pi covalent bond will be formed by lateral overlap, or shoulder-to-shoulder overlap. In this case + orbital overlaps with – orbital, and vice versa. This leads to destructive overlap and formation of antibonding pi bonds.
D)

In this case also pi bonds will be formed as both p and d orbitals are perpendicular to the axis. In this case + orbital overlaps with – orbital, and vice versa. This leads to destructive overlap and formation of antibonding pi bonds.
Therefore, we can conclude that option (B) is the correct answer.
Note:
If $s$ orbital combines with a $p$ orbital perpendicular to the plane, no bond formation takes place and this forms a NBMO (non-bonding molecular orbital). Similarly with two p-orbitals, one in axis and one perpendicular to the axis, for example ${p_x}\& {p_y}$ orbitals on x-axis, will lead to a formation of NBMO.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




