
Which of the following IUPAC names is correct?
A. 2-methyl-3-ethyl pentane
B. 2-ethyl-3-methyl pentane
C. 3-ethyl-2-methyl pentene
D. 3-methyl-2ethyl methylpentane
Answer
561k+ views
Hint: According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete answer:
- In the question it is asked which IUPAC name is correct among the given options.
- From the given IUPAC names in the options it is confirmed that the longest chain contains 5 carbon atoms (penta).
- First we draw the structures of the given options.
- Coming to given options, structure of option A is as follows.
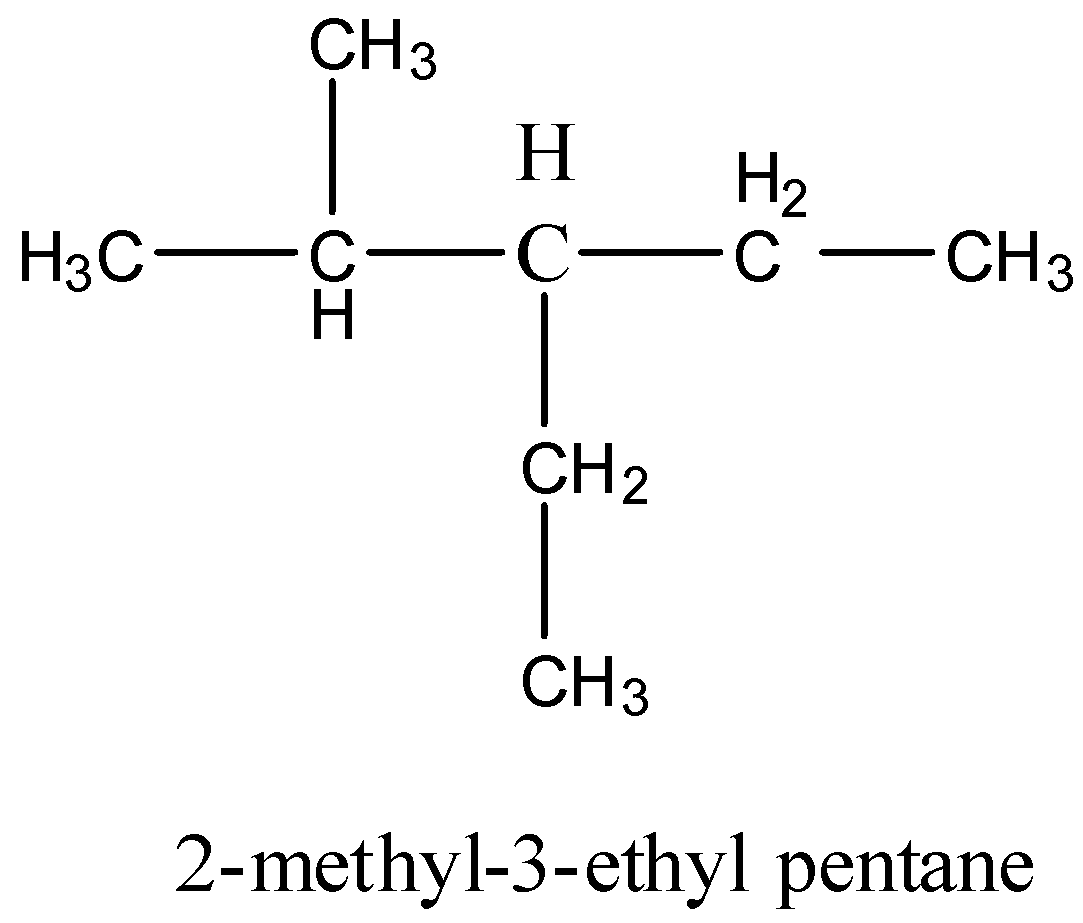
- The given IUPAC name is going to suit the above structure.
- Coming to the option B, 2-ethyl-3-methyl pentane. Structure of the option B.
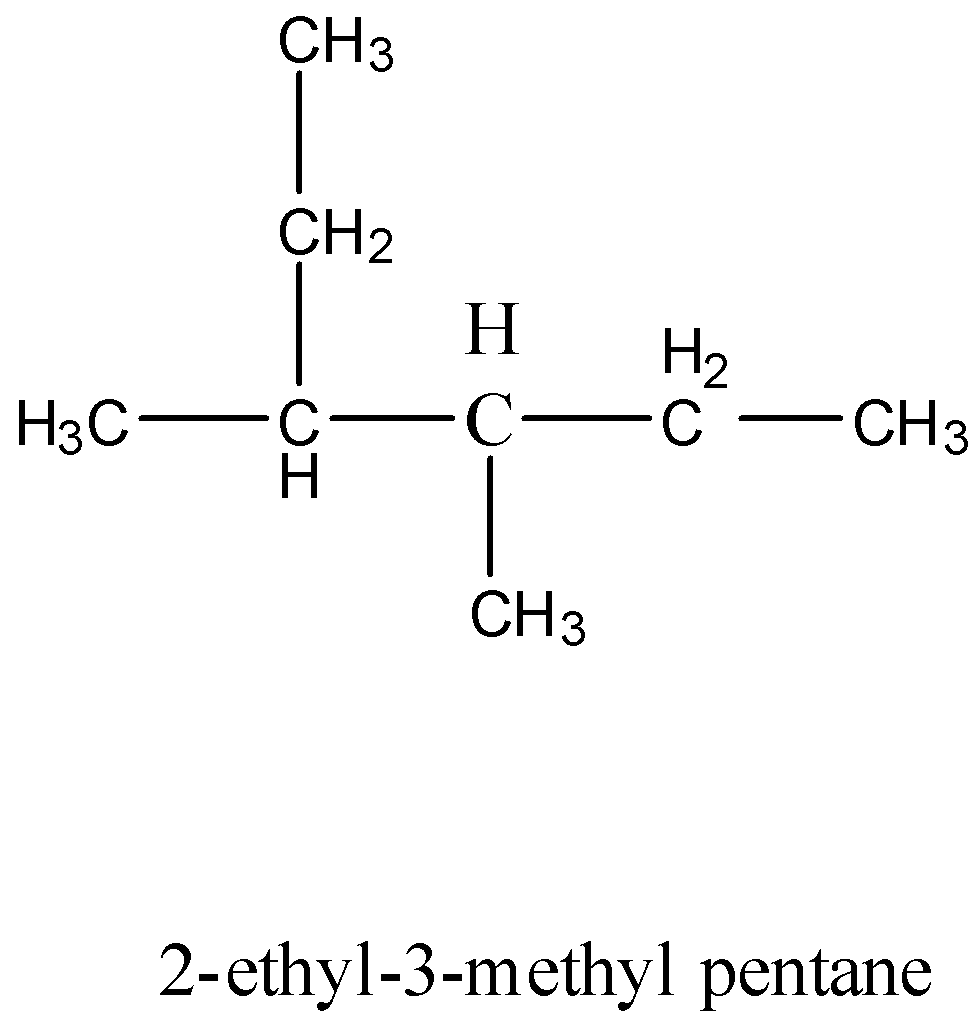
- The IUPAC name is not going to suit option B, because in the above structure the longest chain has six carbons, then the name should hexa. But in the given option it is ‘penta’.
- So, IUPAC name in option b is wrong.
- Coming to the option C, 3-ethyl-2-methyl pentene. The given IUPAC name is wrong, because as per the IUPAC name, the position of the double bond should be mentioned in it. But it is not mentioned so option C is wrong.
- Coming to the option D, 3-methyl-2ethyl methylpentane. The given IUPAC name is wrong, because as per the IUPAC name, the position of the double bond should be mentioned in it. But it is not mentioned so option D is wrong.
So, the correct IUPAC name is option, ‘A. 2-methyl-3-ethyl pentane’.
Note: In the IUPAC name for the alkenes and alkynes, the unsaturation position must be mentioned otherwise the IUPAC name won’t exist. Always we should give less or least numbers to the functional groups which are present in the chain.
Complete answer:
- In the question it is asked which IUPAC name is correct among the given options.
- From the given IUPAC names in the options it is confirmed that the longest chain contains 5 carbon atoms (penta).
- First we draw the structures of the given options.
- Coming to given options, structure of option A is as follows.

- The given IUPAC name is going to suit the above structure.
- Coming to the option B, 2-ethyl-3-methyl pentane. Structure of the option B.

- The IUPAC name is not going to suit option B, because in the above structure the longest chain has six carbons, then the name should hexa. But in the given option it is ‘penta’.
- So, IUPAC name in option b is wrong.
- Coming to the option C, 3-ethyl-2-methyl pentene. The given IUPAC name is wrong, because as per the IUPAC name, the position of the double bond should be mentioned in it. But it is not mentioned so option C is wrong.
- Coming to the option D, 3-methyl-2ethyl methylpentane. The given IUPAC name is wrong, because as per the IUPAC name, the position of the double bond should be mentioned in it. But it is not mentioned so option D is wrong.
So, the correct IUPAC name is option, ‘A. 2-methyl-3-ethyl pentane’.
Note: In the IUPAC name for the alkenes and alkynes, the unsaturation position must be mentioned otherwise the IUPAC name won’t exist. Always we should give less or least numbers to the functional groups which are present in the chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




