
Which of the following is/are constituent(s) of Liquefied Petroleum Gas (LPG)?
(A) propane
(B) butane
(C) isobutane
(D) All of the above
Answer
600.9k+ views
Hint: The constituents of Liquefied Petroleum Gas (LPG) are the aliphatic straight chain of saturated hydrocarbons with three carbons and aliphatic straight and branched chains of saturated hydrocarbons with four carbons. A small concentration of other hydrocarbons are also present but these are major constituents.
Complete step by step answer:
The constituents of Liquefied Petroleum Gas (LPG) are propane (\[{{C}_{3}}{{H}_{8}}\]), butane (\[{{C}_{4}}{{H}_{10}}\]) and isobutene (2-methyl propane)
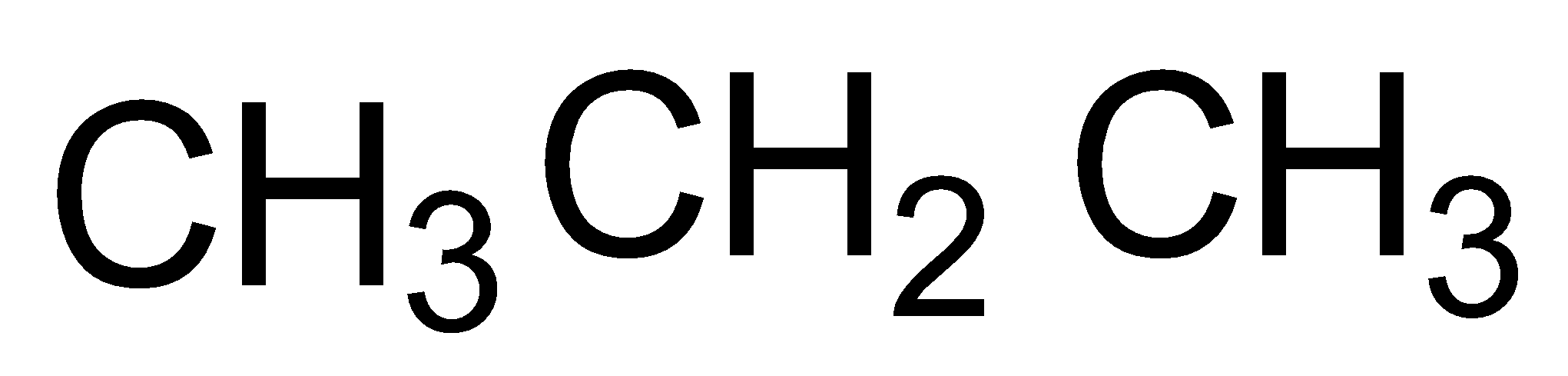
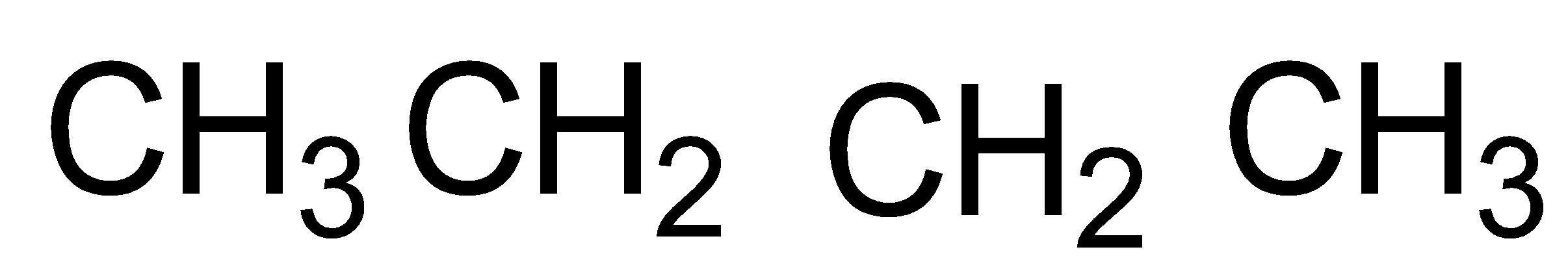
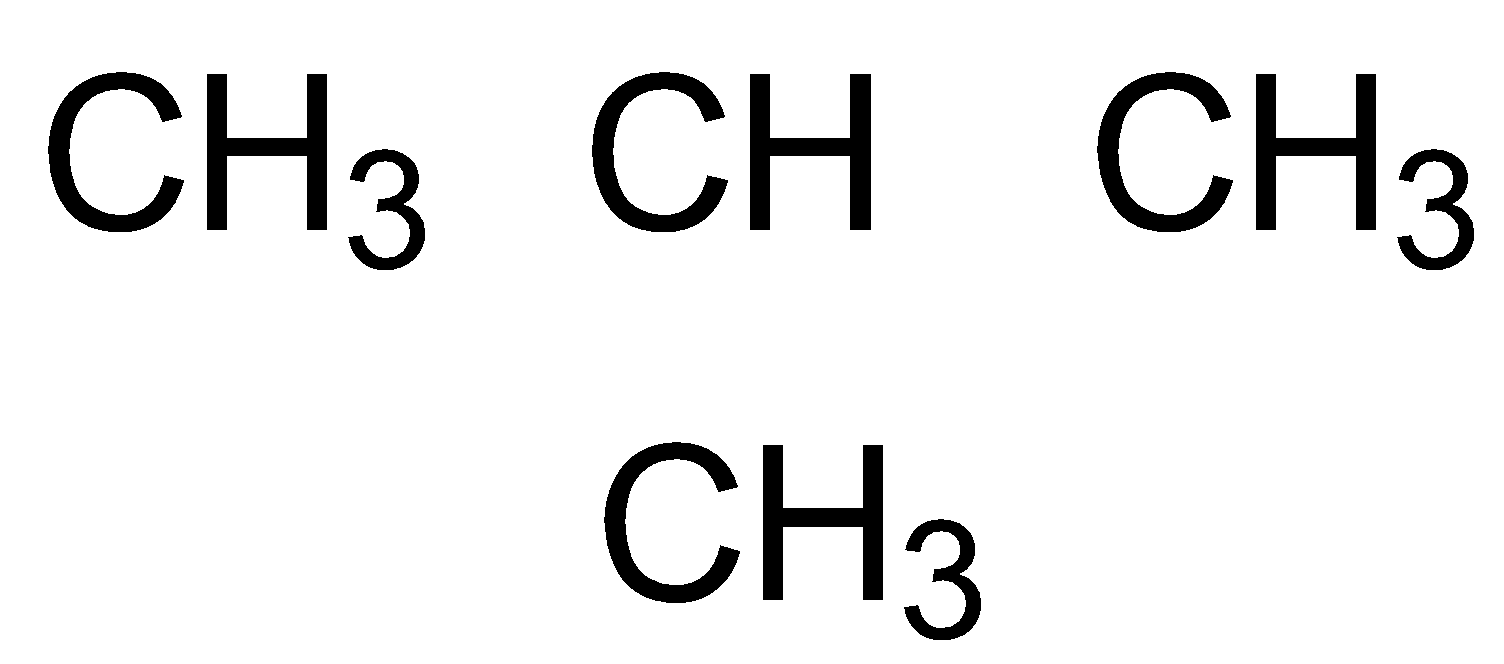
propane butane isobutane
> Percentage of these constituents-
- Propane- 24.5 %
- Butane- 38.5 %
- Isobutane-37 %
- LPG is a highly flammable mixture of these three hydrocarbon gases.
- While propane produces more heat than butane and is more efficient in combustion, butane has an environment-friendly. It liquefies easily, making containment easy.
- Both gases have no long-term adverse effects on the environment. They are safe, non-toxic, clean-burning fuels which are a great source of energy.
- With a lower carbon content than oil, gasoline, diesel, kerosene and ethanol, these gases contain very less greenhouse gas emissions per productivity unit compared to other fuels.
- Natural gas processing and petroleum refining give LPG as a by-product. It is a non-renewable source of energy. The components of LPG are gases at normal temperature and pressure.
- LPG is commonly used as cooking fuel and engine fuel.
- It is non-toxic and non-corrosive in nature. It burns at a much higher calorific value.
So, the correct option is D.
Note: Calorific value is the rate at which a gas/fuel burns.
LPG is environmentally friendly than other forms of energy. LPG is stored under pressure as a liquid in a gas bottle. It is also known as “bottled gas”.
Complete step by step answer:
The constituents of Liquefied Petroleum Gas (LPG) are propane (\[{{C}_{3}}{{H}_{8}}\]), butane (\[{{C}_{4}}{{H}_{10}}\]) and isobutene (2-methyl propane)
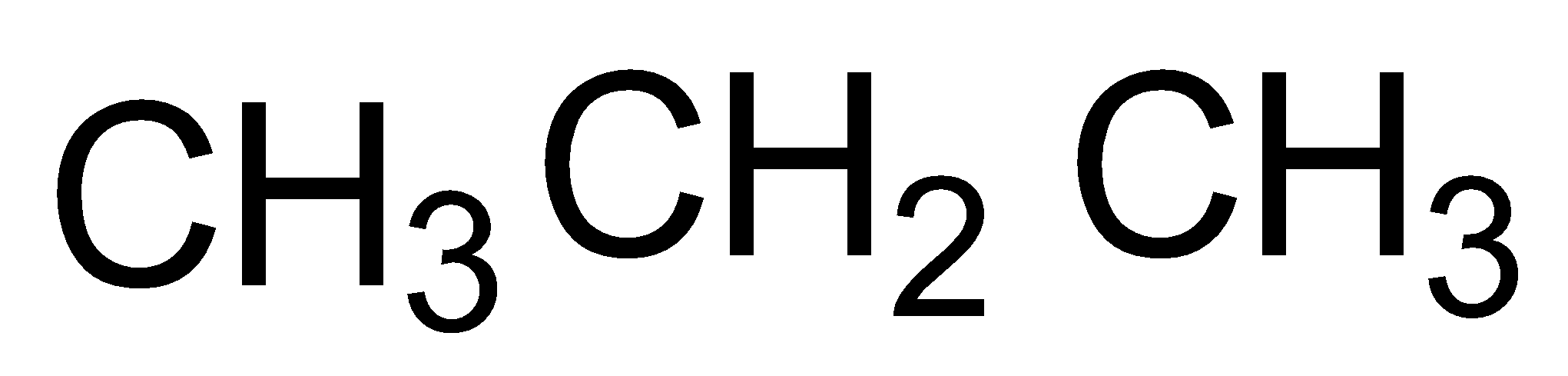
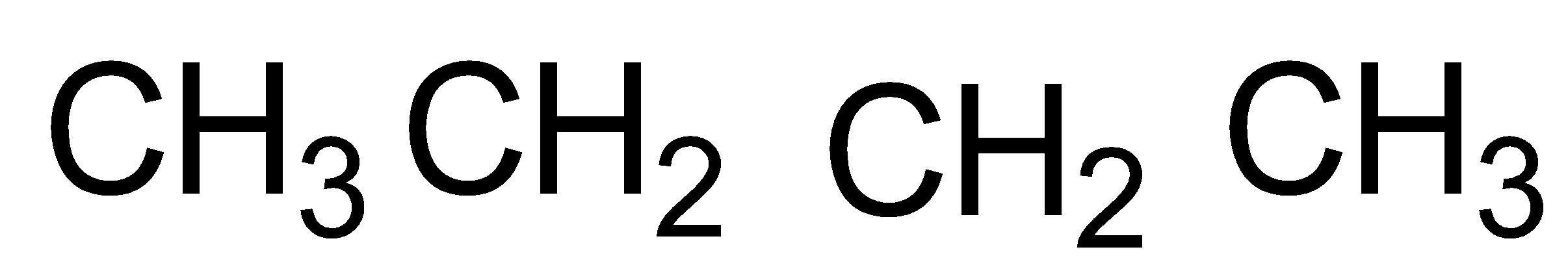

propane butane isobutane
> Percentage of these constituents-
- Propane- 24.5 %
- Butane- 38.5 %
- Isobutane-37 %
- LPG is a highly flammable mixture of these three hydrocarbon gases.
- While propane produces more heat than butane and is more efficient in combustion, butane has an environment-friendly. It liquefies easily, making containment easy.
- Both gases have no long-term adverse effects on the environment. They are safe, non-toxic, clean-burning fuels which are a great source of energy.
- With a lower carbon content than oil, gasoline, diesel, kerosene and ethanol, these gases contain very less greenhouse gas emissions per productivity unit compared to other fuels.
- Natural gas processing and petroleum refining give LPG as a by-product. It is a non-renewable source of energy. The components of LPG are gases at normal temperature and pressure.
- LPG is commonly used as cooking fuel and engine fuel.
- It is non-toxic and non-corrosive in nature. It burns at a much higher calorific value.
So, the correct option is D.
Note: Calorific value is the rate at which a gas/fuel burns.
LPG is environmentally friendly than other forms of energy. LPG is stored under pressure as a liquid in a gas bottle. It is also known as “bottled gas”.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




