
Which of the following is true for any diprotic acid ${{H}_{2}}X$?
(A) ${{K}_{{{a}_{2}}}}>{{K}_{{{a}_{1}}}}$
(B) ${{K}_{{{a}_{1}}}}>{{K}_{{{a}_{2}}}}$
(C)${{K}_{{{a}_{2}}}}=\dfrac{1}{{{K}_{{{a}_{1}}}}}$
(D) ${{K}_{{{a}_{2}}}}={{K}_{{{a}_{1}}}}$
Answer
577.5k+ views
Hint: ${{K}_{{{a}_{1}}}}$ and ${{K}_{{{a}_{2}}}}$ are acid constants for polyprotic acids, which may consist of diprotic acids, triprotic acids, etc.
Complete step by step answer:
Let us first understand about the diprotic acids;
Diprotic acid- A diprotic acid is an acid that can donate two hydrogen ions $\left( {{H}^{+}} \right)$ or protons per molecule in an aqueous solution. Another name for diprotic acid is dibasic acid. A diprotic acid is a type of polyprotic acid, which is an acid able to donate more than one proton per molecule.
Several important acids can be classified as polyprotic acids, which can lose more than one ${{H}^{+}}$ ion when they act as Bronsted acids. Diprotic acids, such as sulphuric acid $\left( {{H}_{2}}S{{O}_{4}} \right)$, carbonic acid $\left( {{H}_{2}}C{{O}_{3}} \right)$, hydrogen sulphide $\left( {{H}_{2}}S \right)$, chromium acid $\left( {{H}_{2}}Cr{{O}_{4}} \right)$, and oxalic acid $\left( {{H}_{2}}{{C}_{2}}{{O}_{4}} \right)$ have two acidic atoms. Triprotic acids, such as phosphoric acid $\left( {{H}_{3}}P{{O}_{4}} \right)$ and citric acid $\left( {{C}_{6}}{{H}_{8}}{{O}_{7}} \right)$, have three.
Diprotic acids-
A diprotic acid (here symbolised by ${{H}_{2}}X$) can undergo one or two dissociations depending on $pH$. Dissociation does not happen all at once; each dissociation step has its own ${{K}_{a}}$ value, designated ${{K}_{{{a}_{1}}}}$and ${{K}_{{{a}_{2}}}}$.
${{H}_{2}}X(aq)\rightleftarrows {{H}^{+}}(aq)+H{{X}^{-}}(aq)$ ${{K}_{{{a}_{1}}}}$
$H{{X}^{-}}(aq)\rightleftarrows {{H}^{+}}(aq)+{{X}^{2-}}(aq)$ ${{K}_{{{a}_{2}}}}$
The first dissociation constant is necessarily greater than the second (i.e. ${{K}_{{{a}_{1}}}}>{{K}_{{{a}_{2}}}}$); this is because the first proton to dissociate is always the most strongly acidic, followed in order by the next most strongly acidic proton.
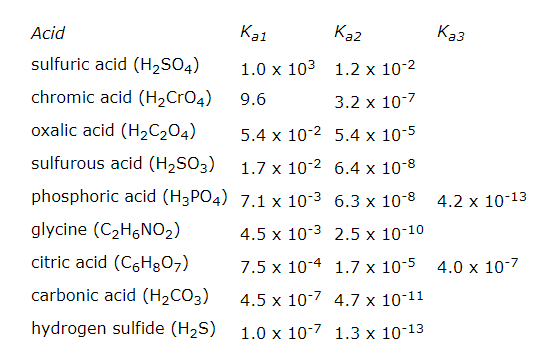
Let us see for the sulphuric acid, who can donate two protons in the solution;
${{H}_{2}}S{{O}_{4}}(aq)\to {{H}^{+}}(aq)+HS{{O}_{4}}^{-}(aq)$ ${{K}_{{{a}_{1}}}}$ = large$HS{{O}_{4}}^{-}(aq)\rightleftarrows {{H}^{+}}(aq)+S{{O}_{4}}^{-}(aq)$ ${{K}_{{{a}_{2}}}}$ = small
This first dissociation step of sulphuric acid will occur completely, which is why sulphuric acid is considered as strong acid; the second dissociation step is only weakly dissociating, however.
So, generally for any polyprotic acid, the first acid constant will always be greater than rest as after losing one proton it becomes difficult to lose another electron and act as acid.
So, the correct answer is “Option B”.
Note: Do note that the trend follows for any polyprotic acid. It’s not limited to only diprotic acids.
The dissociation constant trend is the same for all diprotic acids and this follows for other polyprotic acids too.
Complete step by step answer:
Let us first understand about the diprotic acids;
Diprotic acid- A diprotic acid is an acid that can donate two hydrogen ions $\left( {{H}^{+}} \right)$ or protons per molecule in an aqueous solution. Another name for diprotic acid is dibasic acid. A diprotic acid is a type of polyprotic acid, which is an acid able to donate more than one proton per molecule.
Several important acids can be classified as polyprotic acids, which can lose more than one ${{H}^{+}}$ ion when they act as Bronsted acids. Diprotic acids, such as sulphuric acid $\left( {{H}_{2}}S{{O}_{4}} \right)$, carbonic acid $\left( {{H}_{2}}C{{O}_{3}} \right)$, hydrogen sulphide $\left( {{H}_{2}}S \right)$, chromium acid $\left( {{H}_{2}}Cr{{O}_{4}} \right)$, and oxalic acid $\left( {{H}_{2}}{{C}_{2}}{{O}_{4}} \right)$ have two acidic atoms. Triprotic acids, such as phosphoric acid $\left( {{H}_{3}}P{{O}_{4}} \right)$ and citric acid $\left( {{C}_{6}}{{H}_{8}}{{O}_{7}} \right)$, have three.
Diprotic acids-
A diprotic acid (here symbolised by ${{H}_{2}}X$) can undergo one or two dissociations depending on $pH$. Dissociation does not happen all at once; each dissociation step has its own ${{K}_{a}}$ value, designated ${{K}_{{{a}_{1}}}}$and ${{K}_{{{a}_{2}}}}$.
${{H}_{2}}X(aq)\rightleftarrows {{H}^{+}}(aq)+H{{X}^{-}}(aq)$ ${{K}_{{{a}_{1}}}}$
$H{{X}^{-}}(aq)\rightleftarrows {{H}^{+}}(aq)+{{X}^{2-}}(aq)$ ${{K}_{{{a}_{2}}}}$
The first dissociation constant is necessarily greater than the second (i.e. ${{K}_{{{a}_{1}}}}>{{K}_{{{a}_{2}}}}$); this is because the first proton to dissociate is always the most strongly acidic, followed in order by the next most strongly acidic proton.

Let us see for the sulphuric acid, who can donate two protons in the solution;
${{H}_{2}}S{{O}_{4}}(aq)\to {{H}^{+}}(aq)+HS{{O}_{4}}^{-}(aq)$ ${{K}_{{{a}_{1}}}}$ = large$HS{{O}_{4}}^{-}(aq)\rightleftarrows {{H}^{+}}(aq)+S{{O}_{4}}^{-}(aq)$ ${{K}_{{{a}_{2}}}}$ = small
This first dissociation step of sulphuric acid will occur completely, which is why sulphuric acid is considered as strong acid; the second dissociation step is only weakly dissociating, however.
So, generally for any polyprotic acid, the first acid constant will always be greater than rest as after losing one proton it becomes difficult to lose another electron and act as acid.
So, the correct answer is “Option B”.
Note: Do note that the trend follows for any polyprotic acid. It’s not limited to only diprotic acids.
The dissociation constant trend is the same for all diprotic acids and this follows for other polyprotic acids too.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




