
Which of the following is the correct order of stability of free radicals?
A) Benzyl > allyl > \[2^\circ \] > \[1^\circ \]
B) Allyl > benzyl > \[2^\circ \] > \[1^\circ \]
C) Allyl > \[2^\circ \] > \[1^\circ \] > benzyl
D) benzyl > \[2^\circ \] > \[1^\circ \] > allyl
Answer
512.4k+ views
Hint: We must have to know that in organic chemistry stability of molecules and ions are very important. Carbon ions are classified as two types. There is carbocation and carbanion. The carbon atom which has a positive charge is called carbocation. The carbon atom which has negative charge is called carbocation. In organic chemistry cleavage is split into two types. There are homolytic cleavage and heterolytic cleavage. Ions will happen in heterolytic cleavage. Free radicals will come in Homolytic cleavage.
Complete answer:
The given free radicals are
Benzyl, allyl,\[1^\circ \], \[2^\circ \]
The primary free radical representation is \[1^\circ \].
The secondary free radical representation is \[2^\circ \].
The structure of benzyl free radicals is
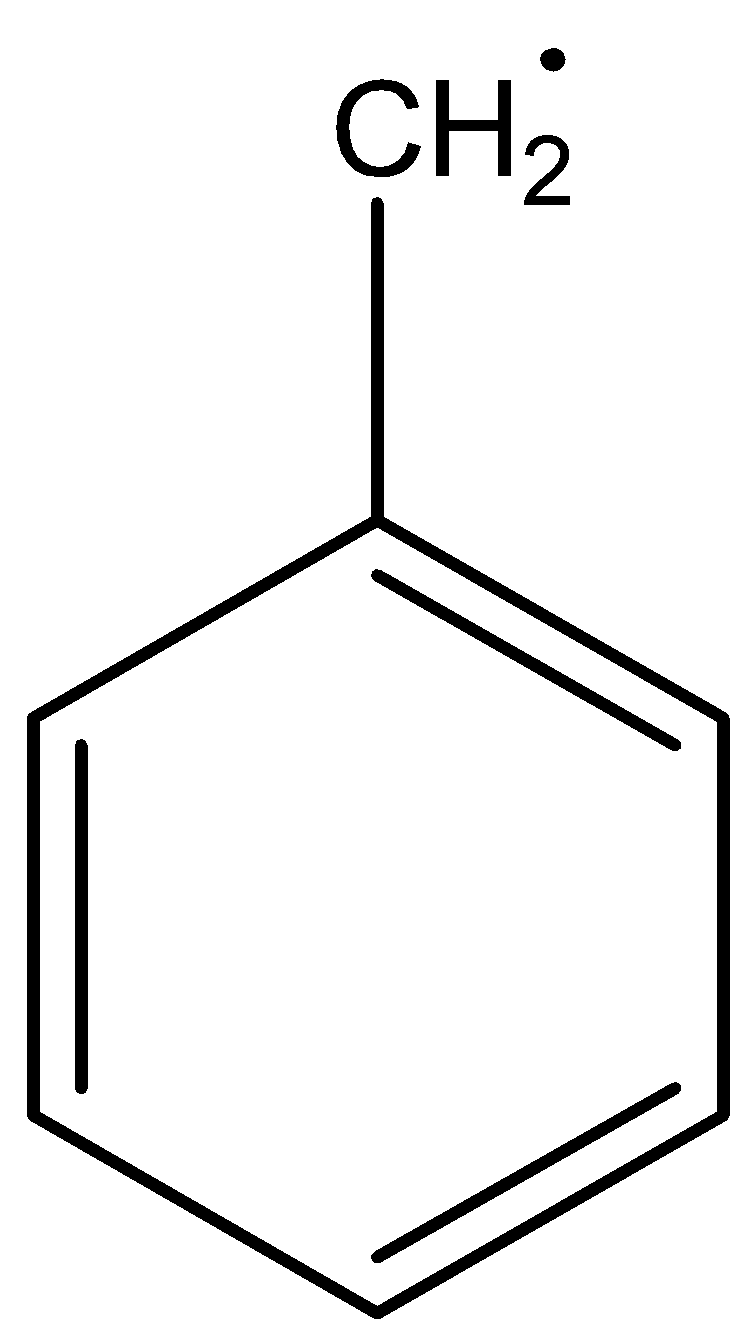
The structure of allyl free radicals is
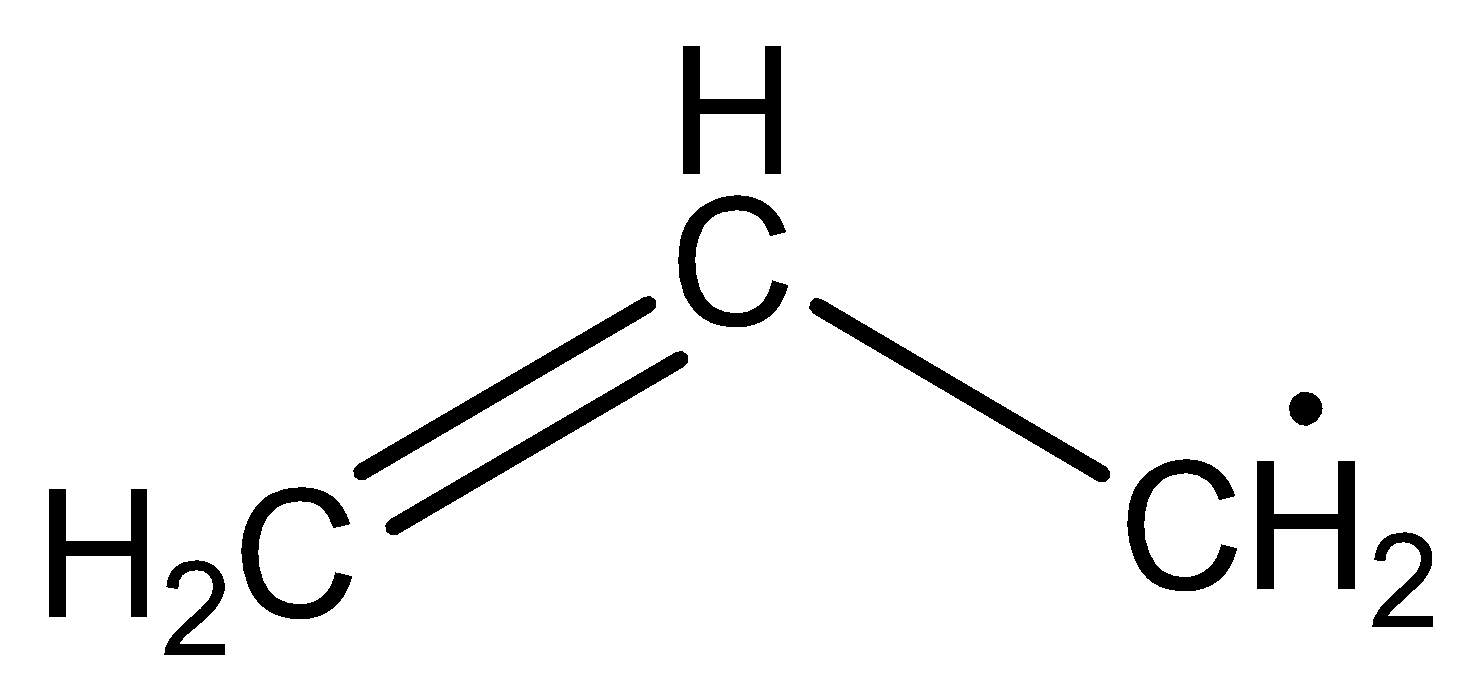
The structure of primary free radicals is
\[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{.}}\]
The structure of secondary free radicals is
\[{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C}}{{\text{H}}^{\text{.}}}\]
Benzyl free radicals are most stable in the given series.
Primary free radicals are most stable in the given series.
According to the above discussion, we conclude the order of stability of free radicals is
Benzyl > allyl > \[2^\circ \] > \[1^\circ \]
Hence, option A is the correct answer.
Note:
We must have to remember that benzyl free radicals are more stable than primary free radicals. Benzyl free radicals are more stable than allyl free radicals. Benzyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than primary free radicals. Secondary free radicals are more stable than primary free radicals.
Complete answer:
The given free radicals are
Benzyl, allyl,\[1^\circ \], \[2^\circ \]
The primary free radical representation is \[1^\circ \].
The secondary free radical representation is \[2^\circ \].
The structure of benzyl free radicals is

The structure of allyl free radicals is

The structure of primary free radicals is
\[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{.}}\]
The structure of secondary free radicals is
\[{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C}}{{\text{H}}^{\text{.}}}\]
Benzyl free radicals are most stable in the given series.
Primary free radicals are most stable in the given series.
According to the above discussion, we conclude the order of stability of free radicals is
Benzyl > allyl > \[2^\circ \] > \[1^\circ \]
Hence, option A is the correct answer.
Note:
We must have to remember that benzyl free radicals are more stable than primary free radicals. Benzyl free radicals are more stable than allyl free radicals. Benzyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than primary free radicals. Secondary free radicals are more stable than primary free radicals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




