
Which of the following is steam volatile?
A. Nitrobenzene
B. Aniline
C. Both A and B
D. Neither A nor B
Answer
569.7k+ views
Hint: Steam volatility is related to the boiling point of liquids. A substance can be steam volatile if it is easily evaporated on passing steam through it. The causes of this can be forces of attraction between the molecule and water, such as hydrogen bonding
Complete step by step answer:
Substances or compounds which distil with steam in steam distillation conditions are known as steam volatile compounds.
Let us have a look at what happens during steam distillation:
First, water is heated in a beaker and made to boil. The resulting steam is then allowed to mix with the compound, kept in a separate beaker. Substances which are steam volatile have low boiling points, and thus, get vapourized when contacted with the high temperature steam.
Let us have a look at a possible reason as to why compounds may be steam volatile:
One such cause is hydrogen bonding, which is an interaction between hydrogen atom and nitrogen/oxygen/fluorine atoms present on the same molecule or on another molecule. As we know, nitrobenzene contains a nitrogen atom, and this can form hydrogen bonds with the hydrogen from steam. Its structure is shown below:
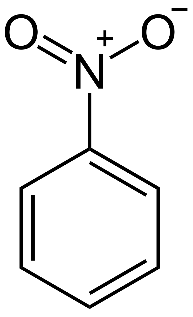
Aniline too has a nitrogen atom, and thus, it too forms hydrogen bonds with steam. Aniline is shown in the figure below:
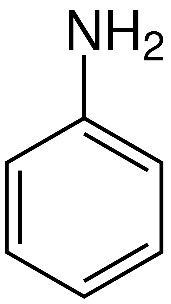
This makes it easier for the steam to carry away these compounds as vapour, thus making them both steam volatile.
Hence, since both compounds are steam volatile
So, the correct answer is Option C.
Note: When steam is introduced into a container having the compound, it increases the vapour pressure of the mixture. As we know, a liquid boils when its vapour pressure becomes equal to the external pressure. The hydrogen bonding helps in the formation of a good vapour mixture, whose vapour pressure is increased due to the addition of steam. Thus, the mixture boils and is passed on as a vapour mixture.
Complete step by step answer:
Substances or compounds which distil with steam in steam distillation conditions are known as steam volatile compounds.
Let us have a look at what happens during steam distillation:
First, water is heated in a beaker and made to boil. The resulting steam is then allowed to mix with the compound, kept in a separate beaker. Substances which are steam volatile have low boiling points, and thus, get vapourized when contacted with the high temperature steam.
Let us have a look at a possible reason as to why compounds may be steam volatile:
One such cause is hydrogen bonding, which is an interaction between hydrogen atom and nitrogen/oxygen/fluorine atoms present on the same molecule or on another molecule. As we know, nitrobenzene contains a nitrogen atom, and this can form hydrogen bonds with the hydrogen from steam. Its structure is shown below:

Aniline too has a nitrogen atom, and thus, it too forms hydrogen bonds with steam. Aniline is shown in the figure below:

This makes it easier for the steam to carry away these compounds as vapour, thus making them both steam volatile.
Hence, since both compounds are steam volatile
So, the correct answer is Option C.
Note: When steam is introduced into a container having the compound, it increases the vapour pressure of the mixture. As we know, a liquid boils when its vapour pressure becomes equal to the external pressure. The hydrogen bonding helps in the formation of a good vapour mixture, whose vapour pressure is increased due to the addition of steam. Thus, the mixture boils and is passed on as a vapour mixture.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




