
Which of the following is paramagnetic?
A. $CO$
B. ${{O}_{2}}^{-}$
C. $C{{N}^{-}}$
D. $N{{O}^{+}}$
Answer
558.9k+ views
Hint: A molecule having one or more unpaired electrons in their valence shell is paramagnetic or having an odd electron system is paramagnetic. As an example, the answer compound has 15 electrons and encompasses a bond order of two. 5 and has 1 unpaired electron in ${{\pi }^{*}}$ antibonding orbitals. Therefore could be a paramagnetic molecule. we'd like to grasp about molecular orbital theory in detail for further clarification.
Complete step by step answer:
- There are 3 types of magnetic substances: paramagnetic, diamagnetic, ferromagnetic substances. Paramagnetic substances are weakly attracted by magnets, diamagnetic substances are repelled by magnets and ferromagnetic substances are strongly attracted by magnets.
- Molecular orbitals are obtained by combining the atomic orbitals on the atoms within the molecule. Consider the molecule $N{{O}_{2}}$, for instance. one amongst the molecular orbitals during this molecule is built by adding the mathematical functions for both the 1s atomic orbitals that move to create this molecule. Another orbital is created by subtracting one in every of these functions from the opposite.
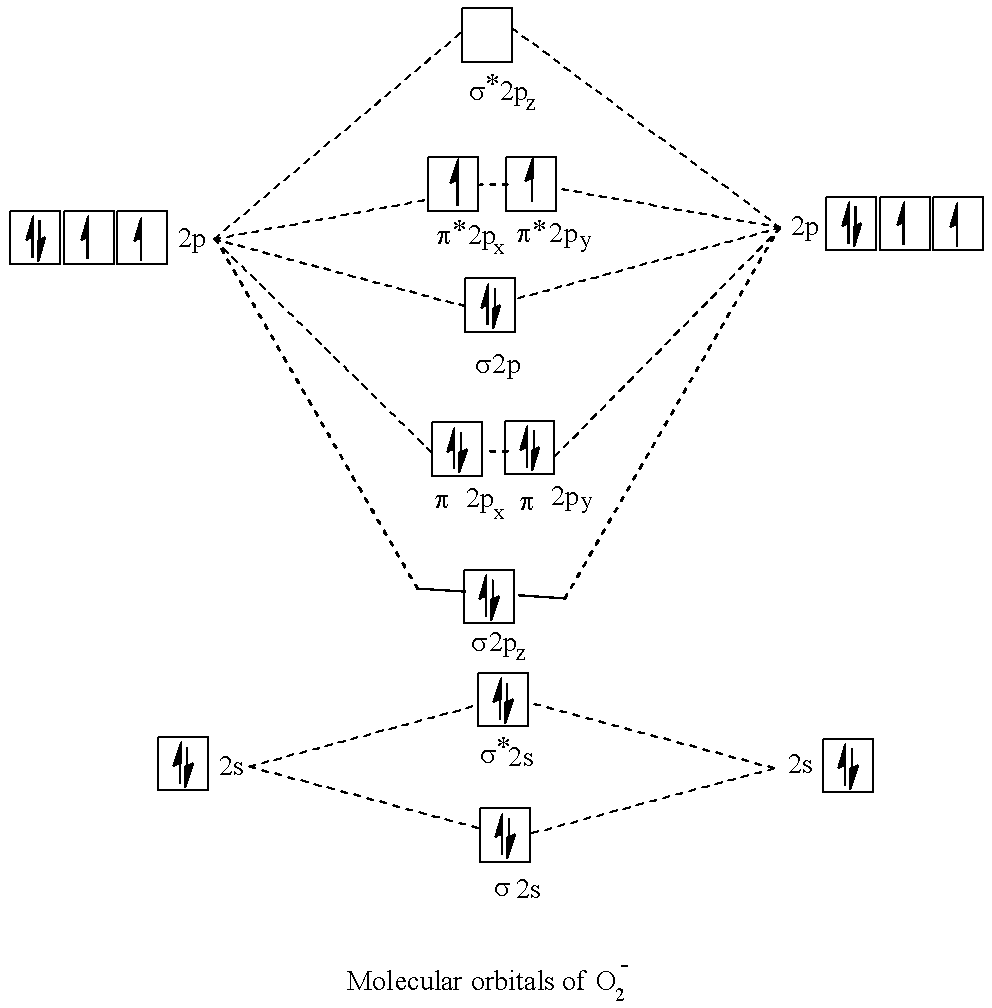
- Molecular orbital (MO) theory describes the behavior of electrons in an exceedingly large molecule in terms of combinations of the atomic wave functions. Materials with unpaired electrons or odd numbered electrons are paramagnetic and drawn to a magnetic field, while those with all-paired electrons that's even no of electrons are diamagnetic and repelled by a magnetic force field. Now let's come to the answer part.
- $CO$,$C{{N}^{-}}$ and $N{{O}^{+}}$ are isoelectronic with 14 electrons each and there's no unpaired electrons present within the MO configuration of those species. So these are diamagnetic. $N{{O}^{+}}$ is paramagnetic in nature thanks to the presence of 1 unpaired electron within the valence shell.
So the correct answer is “B”:
Note: A simple rule of thumb is employed in chemistry to work out whether a particle (atom, ion, or molecule) is paramagnetic or diamagnetic in nature. If all the electrons within the particle are paired, then the substance product of this particle is diamagnetic, If it's unpaired electrons, then the substance is paramagnetic in nature.
Complete step by step answer:
- There are 3 types of magnetic substances: paramagnetic, diamagnetic, ferromagnetic substances. Paramagnetic substances are weakly attracted by magnets, diamagnetic substances are repelled by magnets and ferromagnetic substances are strongly attracted by magnets.
- Molecular orbitals are obtained by combining the atomic orbitals on the atoms within the molecule. Consider the molecule $N{{O}_{2}}$, for instance. one amongst the molecular orbitals during this molecule is built by adding the mathematical functions for both the 1s atomic orbitals that move to create this molecule. Another orbital is created by subtracting one in every of these functions from the opposite.

- Molecular orbital (MO) theory describes the behavior of electrons in an exceedingly large molecule in terms of combinations of the atomic wave functions. Materials with unpaired electrons or odd numbered electrons are paramagnetic and drawn to a magnetic field, while those with all-paired electrons that's even no of electrons are diamagnetic and repelled by a magnetic force field. Now let's come to the answer part.
- $CO$,$C{{N}^{-}}$ and $N{{O}^{+}}$ are isoelectronic with 14 electrons each and there's no unpaired electrons present within the MO configuration of those species. So these are diamagnetic. $N{{O}^{+}}$ is paramagnetic in nature thanks to the presence of 1 unpaired electron within the valence shell.
So the correct answer is “B”:
Note: A simple rule of thumb is employed in chemistry to work out whether a particle (atom, ion, or molecule) is paramagnetic or diamagnetic in nature. If all the electrons within the particle are paired, then the substance product of this particle is diamagnetic, If it's unpaired electrons, then the substance is paramagnetic in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




