
Which of the following is oxidation with alkaline \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]followed by acidification with dil. HCl gives terephthalic acid?
A.p-Ethyl toluene
B.n-Butane
C.1,3-Di-isopropyl benzene
D.m-Xylene
Answer
596.4k+ views
Hint: We must know two things to address this question.
Structure of terephthalic acid
And, the role of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]as an oxidizing agent. Then only we find the product of the reaction.
Complete step by step solution:
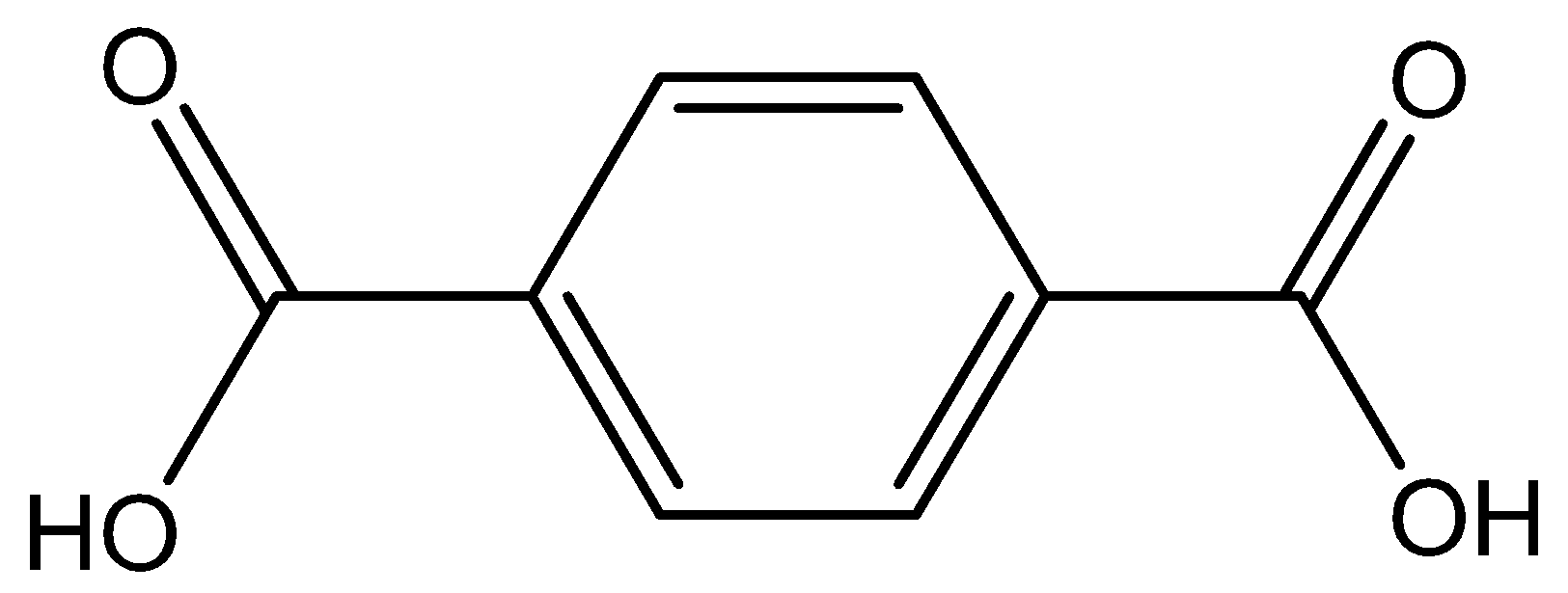
First we must remember that (i) Structure of terephthalic acid: - Terephthalic acid is a benzenedicarboxylic acid with carboxyl groups at positions 1 and 4.
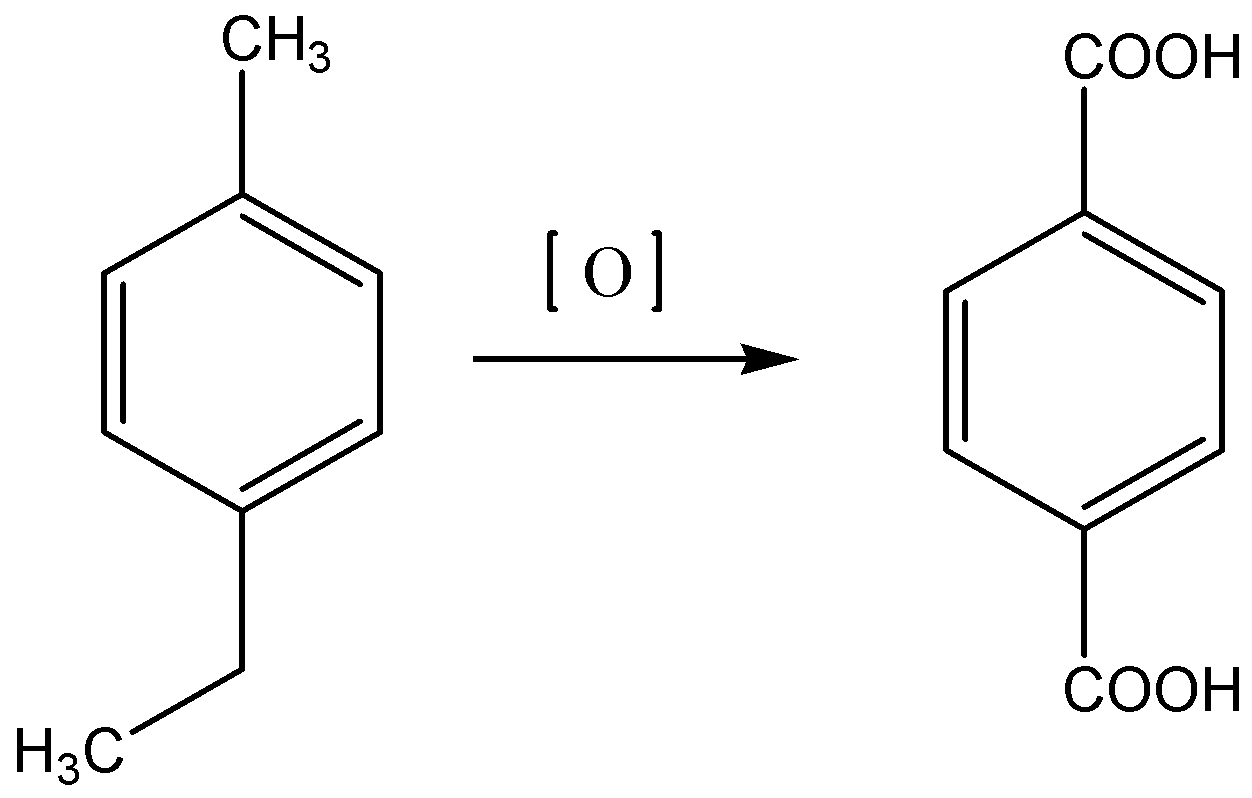
(ii) Role of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]as an oxidising agent in the presence of organic compounds: - The reaction of an alkyl benzene with potassium permanganate (\[{\text{KMn}}{{\text{O}}_{\text{4}}}\]) results in oxidation to benzoic acid. Now, we must note that the position directly adjacent to an aromatic group is called the “benzylic” position, and should contain at least one hydrogen attached to the carbon. For example, in the reaction below you can find all the reactants get converted to carboxylic acid derivatives except for the last one due to the absence of hydrogen at the benzylic position.

Another important point, in order to get the product terephthalic acid, we must look for alkyl groups with at 1 and 4 positions on a benzene ring provided it has at least one hydrogen at benzylic carbon. Only option (A) satisfies these criteria. Let’s look at structure of option A given below:
We can analyse the other options for verification:
Option B: n-Butane is not aromatic so we cannot get an aromatic compound upon oxidation.
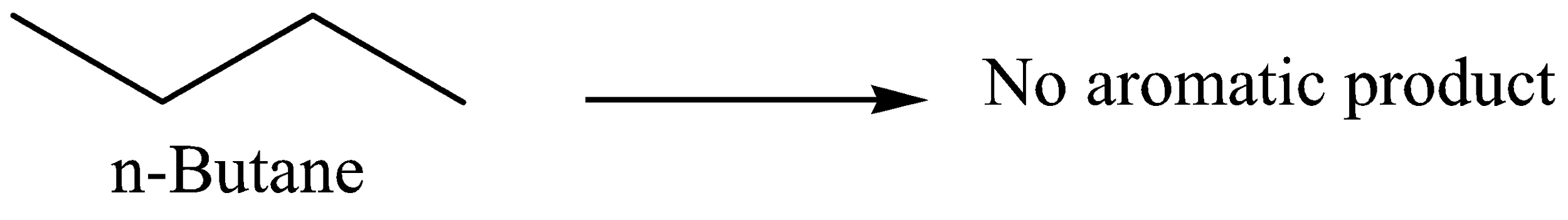
Therefore, it’s an incorrect option.
Option C: 1, 3-Di-isopropyl benzene has alkyl group at 1 and 3 positions, therefore we cannot get the desired product on oxidation.

Option D: m-Xylene also has methyl groups at 1 and 3 positions; therefore we cannot get the desired product on oxidation.

Therefore, we can conclude that Option A is the correct answer among the following.
Note: 1. \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]Oxidises all alkyl group on the benzene ring that has at least one hydrogen on the benzylic carbon.
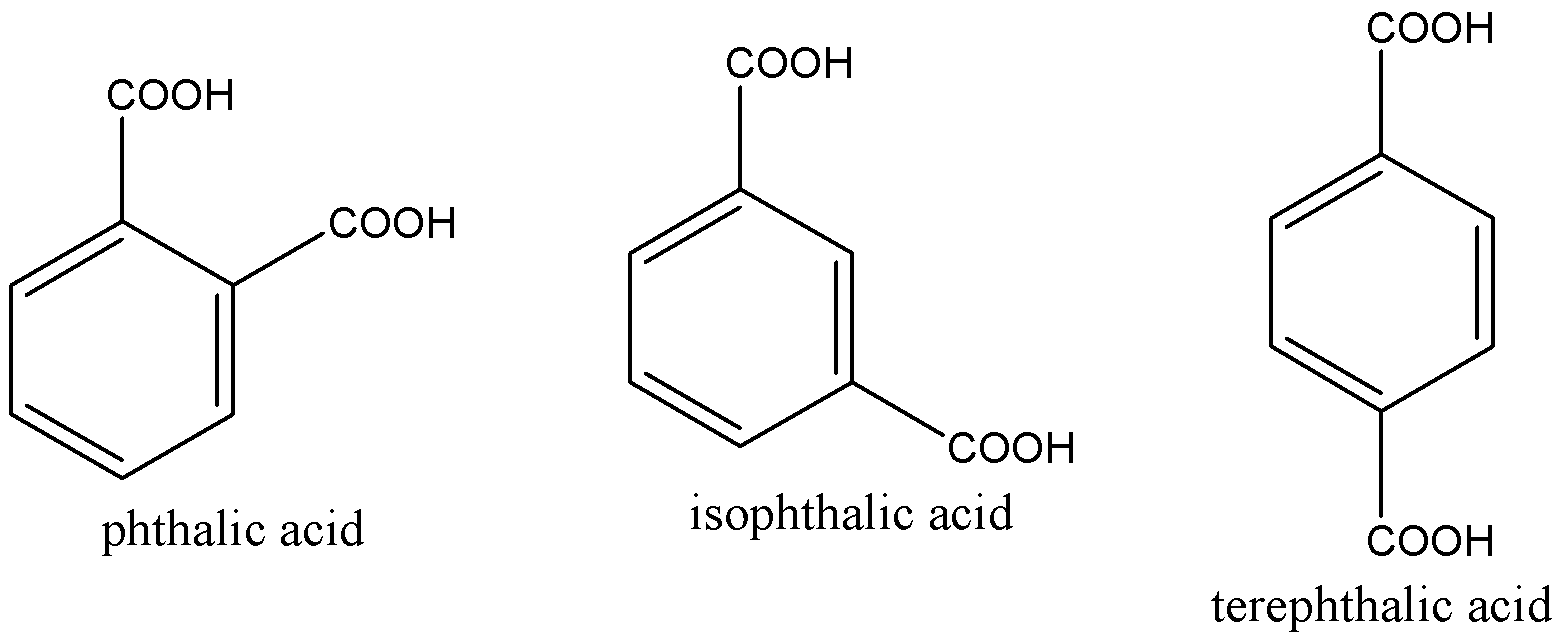
2. Structure of terephthalic should not be confused with other derivatives of benzenedicarboxylic acid. Otherwise the student will end up opting for the wrong answer.
Structure of terephthalic acid
And, the role of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]as an oxidizing agent. Then only we find the product of the reaction.
Complete step by step solution:
First we must remember that (i) Structure of terephthalic acid: - Terephthalic acid is a benzenedicarboxylic acid with carboxyl groups at positions 1 and 4.

(ii) Role of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]as an oxidising agent in the presence of organic compounds: - The reaction of an alkyl benzene with potassium permanganate (\[{\text{KMn}}{{\text{O}}_{\text{4}}}\]) results in oxidation to benzoic acid. Now, we must note that the position directly adjacent to an aromatic group is called the “benzylic” position, and should contain at least one hydrogen attached to the carbon. For example, in the reaction below you can find all the reactants get converted to carboxylic acid derivatives except for the last one due to the absence of hydrogen at the benzylic position.

Another important point, in order to get the product terephthalic acid, we must look for alkyl groups with at 1 and 4 positions on a benzene ring provided it has at least one hydrogen at benzylic carbon. Only option (A) satisfies these criteria. Let’s look at structure of option A given below:

We can analyse the other options for verification:
Option B: n-Butane is not aromatic so we cannot get an aromatic compound upon oxidation.

Therefore, it’s an incorrect option.
Option C: 1, 3-Di-isopropyl benzene has alkyl group at 1 and 3 positions, therefore we cannot get the desired product on oxidation.

Option D: m-Xylene also has methyl groups at 1 and 3 positions; therefore we cannot get the desired product on oxidation.

Therefore, we can conclude that Option A is the correct answer among the following.
Note: 1. \[{\text{KMn}}{{\text{O}}_{\text{4}}}\]Oxidises all alkyl group on the benzene ring that has at least one hydrogen on the benzylic carbon.
2. Structure of terephthalic should not be confused with other derivatives of benzenedicarboxylic acid. Otherwise the student will end up opting for the wrong answer.

Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

How many states of matter are there in total class 12 chemistry CBSE




