
Which of the following is not aromatic in nature?
A.
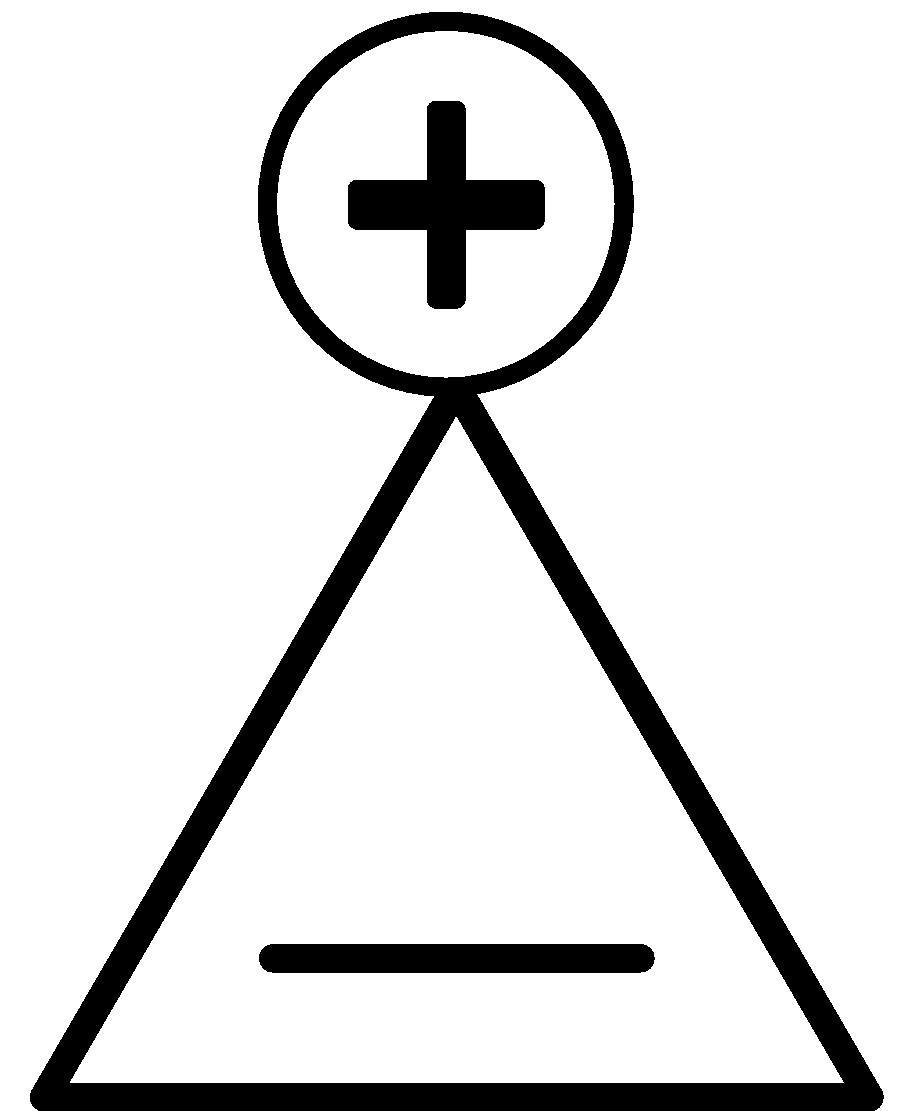
B.
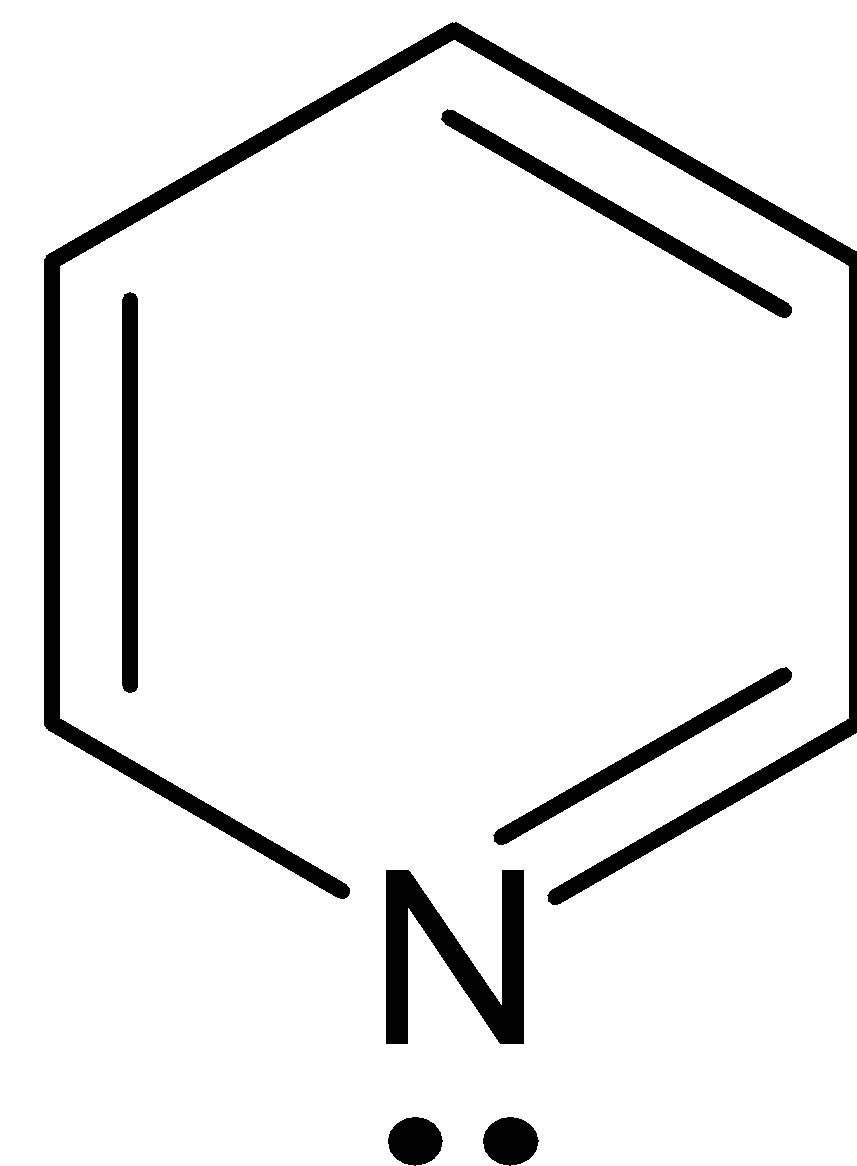
C.
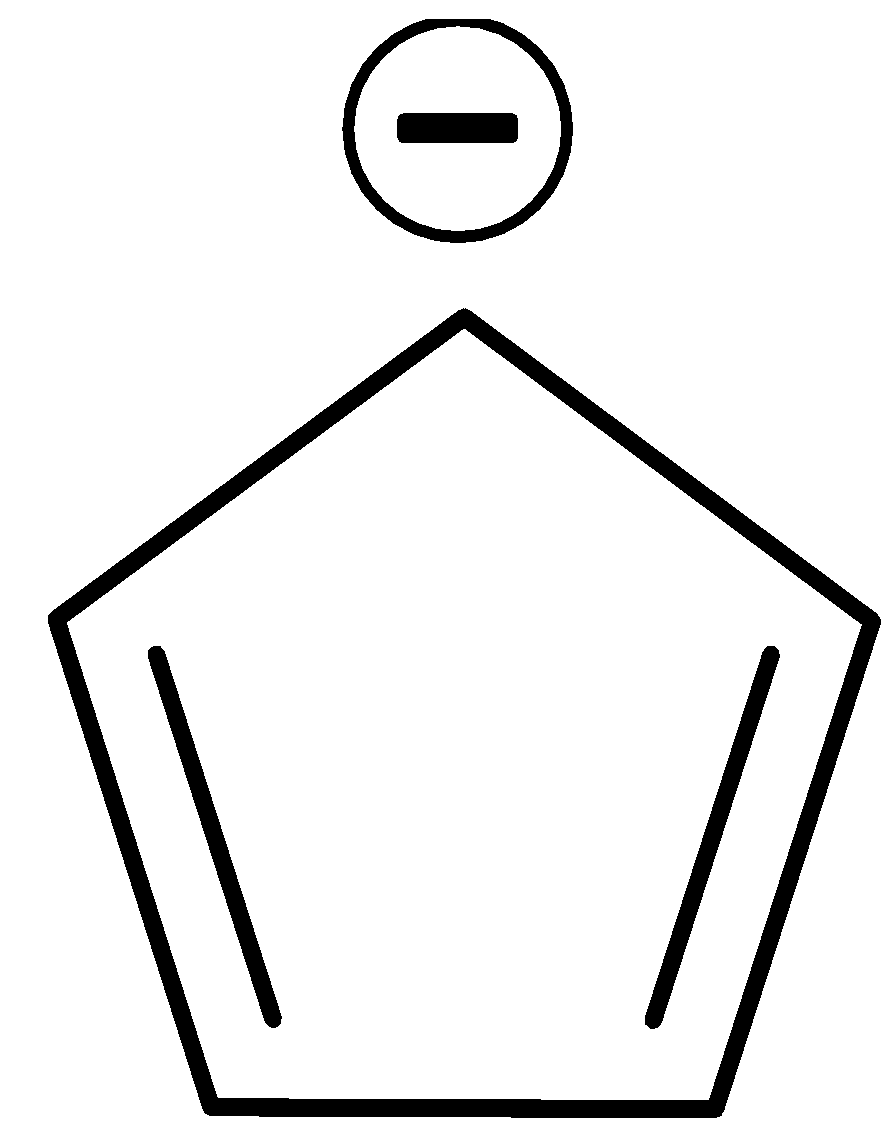
D.
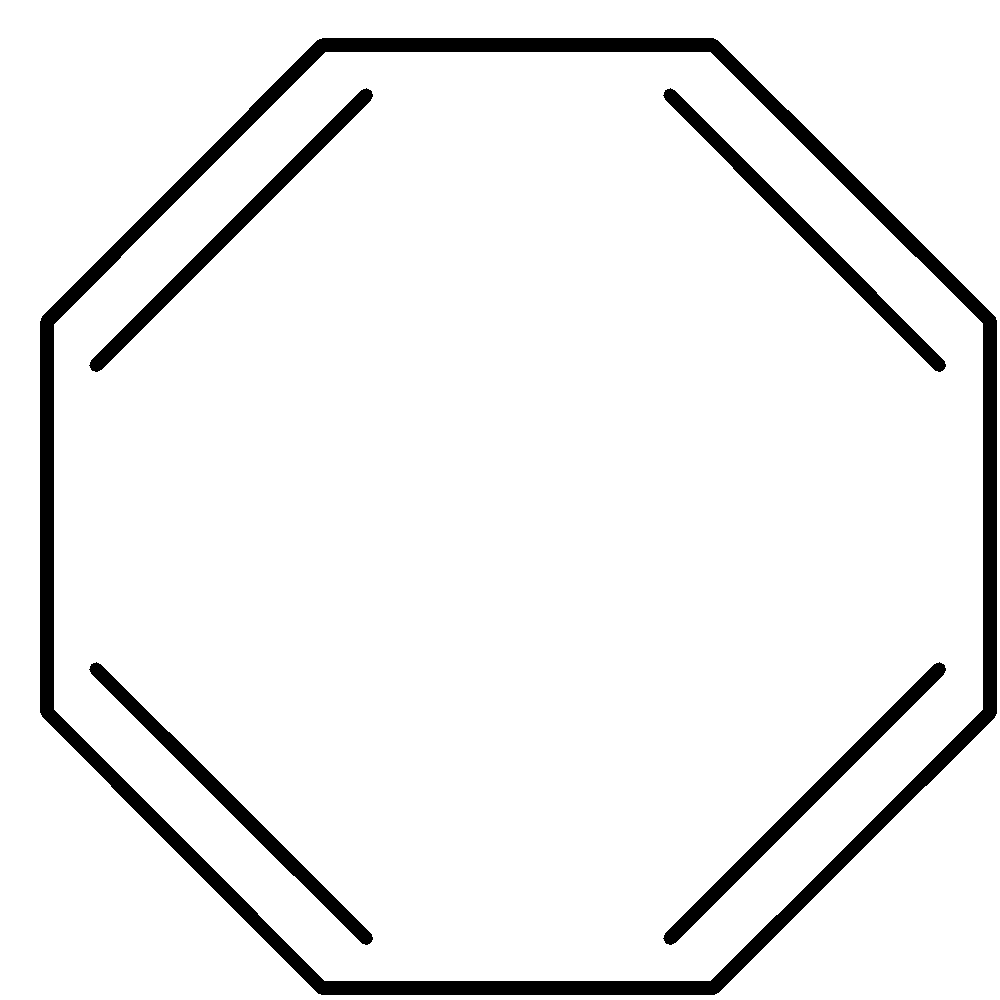




Answer
567k+ views
Hint: Four structural criteria must be satisfied for a compound to be aromatic. The compound must be cyclic, conjugated, planar, and satisfies Huckel’s rule. Electrons in the p orbital are called $\pi $ electrons. Also we can confirm whether an atom is ${{s}}{{{p}}^2}$ hybridized by checking whether the atom has bonded to three atoms and it has no lone pairs.
Complete step by step answer:
Aromatic compounds have a distinctive odor. For a compound to be aromatic, it should be cyclic, planar with resonance bonds, not contain ${{s}}{{{p}}^3}$ hybridized carbon atom and satisfies Huckel’s rule.
When the compound has a ring of atoms, it is called a cyclic compound. It is said to be planar if all the atoms are in the same plane.
Huckel’s rule states that the aromatic compounds contain $\left( {4{{n}} + 2} \right)\pi $ electrons, where ${{n}}$ is any integer.
A.
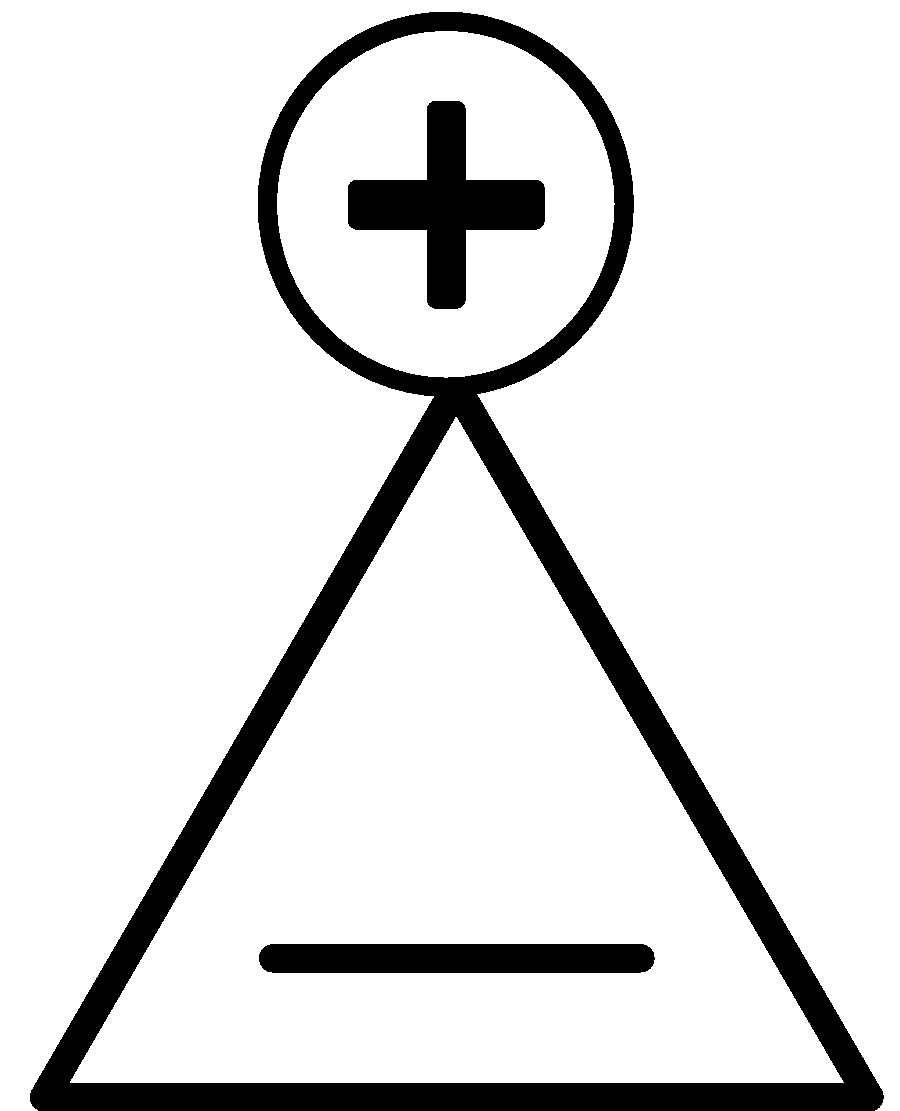
This compound has two $\pi $ electrons. It is planar, cyclic and obeys Huckel’s rule of aromaticity.
$\left( {4{{n}} + 2} \right) = 2 \\
4{{n}} = 0 \\
{{n}} = 0 \\ $
B.
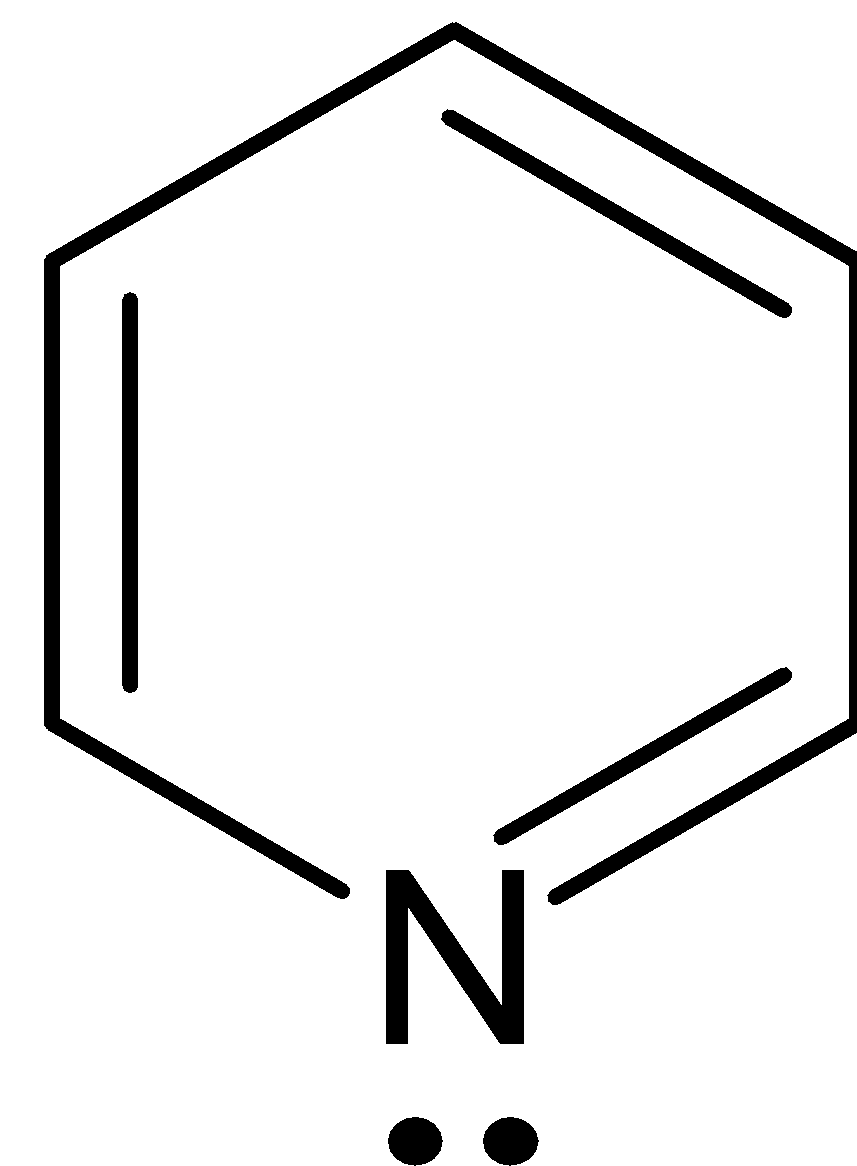
This compound has six $\pi $ electrons, thereby obeys Huckel’s rule where ${{n}} = 1$ and it is cyclic, planar and conjugated.
C.
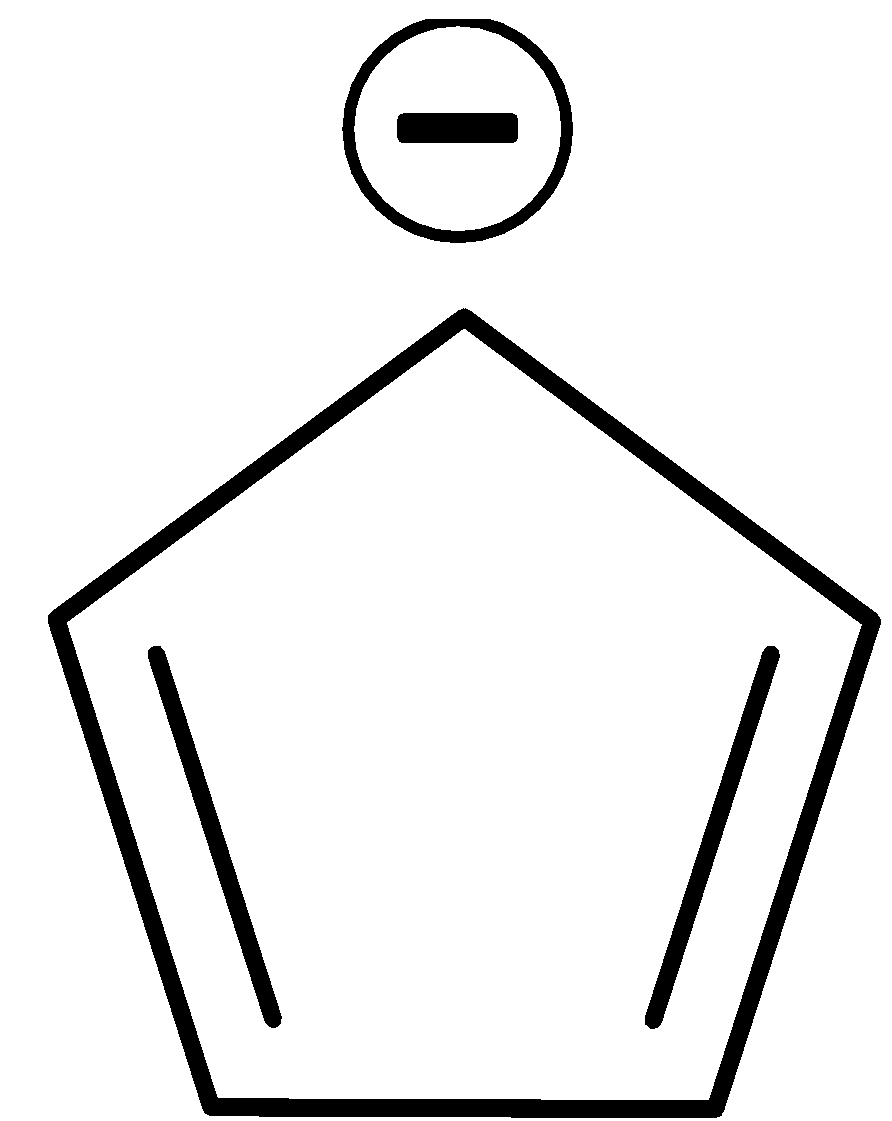
It has six $\pi $ electrons since the negative charge is considered as electrons. Thus it obeys Huckel’s rule. It is also cyclic, planar and conjugated.
D. It has eight $\pi $ electrons, which satisfy $4{{n}}\pi $ rule of anti aromaticity, where ${{n}} = 2$. Thus it is not aromatic, but anti aromatic.
So, the correct answer is Option D.
Note: Based on Huckel’s molecular orbital theory, when all the bonding orbitals are filled with electrons, it is said to be stable. In aromatic compounds, two electrons are filled in the lower energy orbital and four electrons are filled in the following energy level.
Complete step by step answer:
Aromatic compounds have a distinctive odor. For a compound to be aromatic, it should be cyclic, planar with resonance bonds, not contain ${{s}}{{{p}}^3}$ hybridized carbon atom and satisfies Huckel’s rule.
When the compound has a ring of atoms, it is called a cyclic compound. It is said to be planar if all the atoms are in the same plane.
Huckel’s rule states that the aromatic compounds contain $\left( {4{{n}} + 2} \right)\pi $ electrons, where ${{n}}$ is any integer.
A.

This compound has two $\pi $ electrons. It is planar, cyclic and obeys Huckel’s rule of aromaticity.
$\left( {4{{n}} + 2} \right) = 2 \\
4{{n}} = 0 \\
{{n}} = 0 \\ $
B.

This compound has six $\pi $ electrons, thereby obeys Huckel’s rule where ${{n}} = 1$ and it is cyclic, planar and conjugated.
C.

It has six $\pi $ electrons since the negative charge is considered as electrons. Thus it obeys Huckel’s rule. It is also cyclic, planar and conjugated.
D. It has eight $\pi $ electrons, which satisfy $4{{n}}\pi $ rule of anti aromaticity, where ${{n}} = 2$. Thus it is not aromatic, but anti aromatic.
So, the correct answer is Option D.
Note: Based on Huckel’s molecular orbital theory, when all the bonding orbitals are filled with electrons, it is said to be stable. In aromatic compounds, two electrons are filled in the lower energy orbital and four electrons are filled in the following energy level.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




