
Which of the following is not aromatic?
A.Benzene
B.cyclooctatetraenyl dianion
C.tropylium ion
D.cyclopentadienyl cation
Answer
590.1k+ views
Hint: For a molecule to be aromatic, it should be cyclic, planar, must fulfil Huckel’s rule and must be conjugated. His rule states that if a cyclic, planar molecule has 4n+2 π electrons, it is considered To be aromatic.
Complete step by step answer:
Non-aromatic does not follow Huckel’s rule. are highly unstable and highly reactive.
A.Benzene is cyclic, planar and has 6 $\pi $ electrons thus fulfilling Huckel’s rule. It is therefore aromatic.

6 $\pi $ electrons
B.Cyclooctatetraenyl Dianion has 10 $\pi $ electrons thus fulfilling Huckel’s rule. It is cyclic as well as planar. It is therefore aromatic.
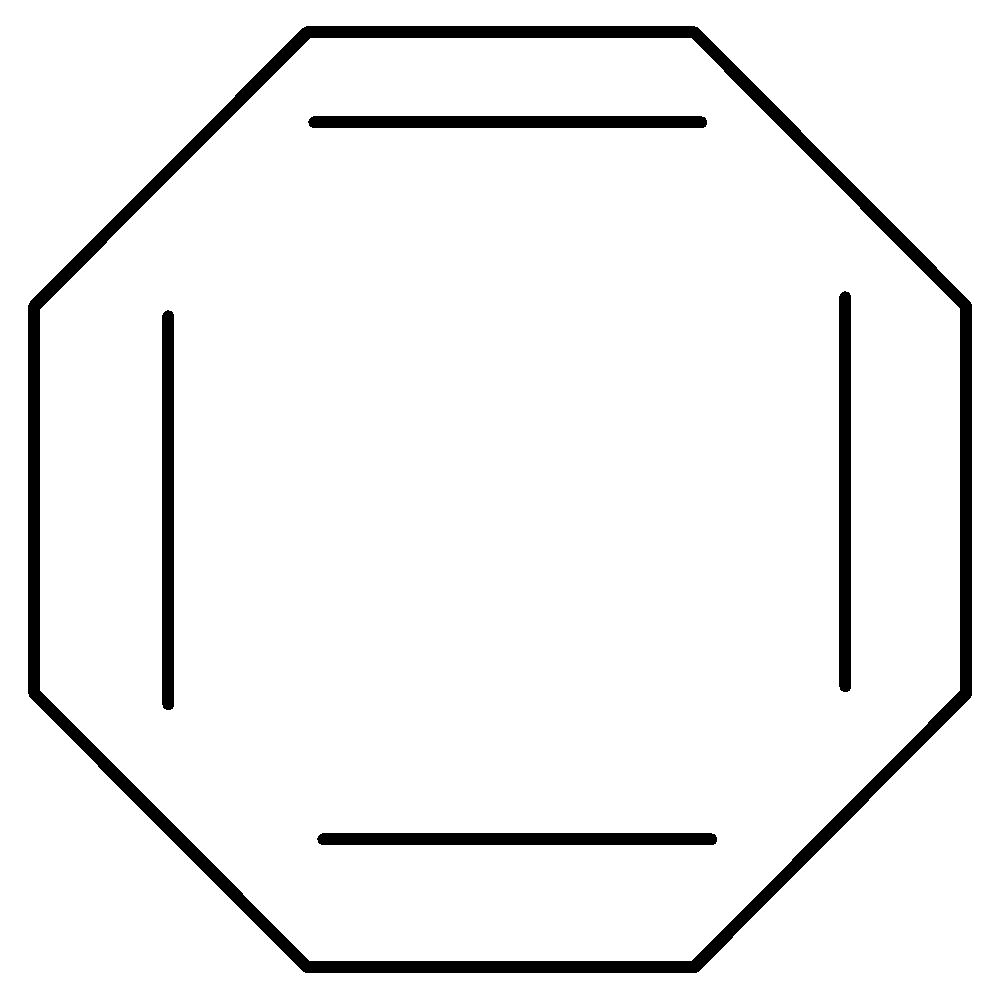
10 $\pi $ electrons
C.Tropylium Ion has 6 $\pi $ electrons. Thus it also fulfils Huckel’s rule. It is cyclic as well as planar. Therefore, it is also aromatic.
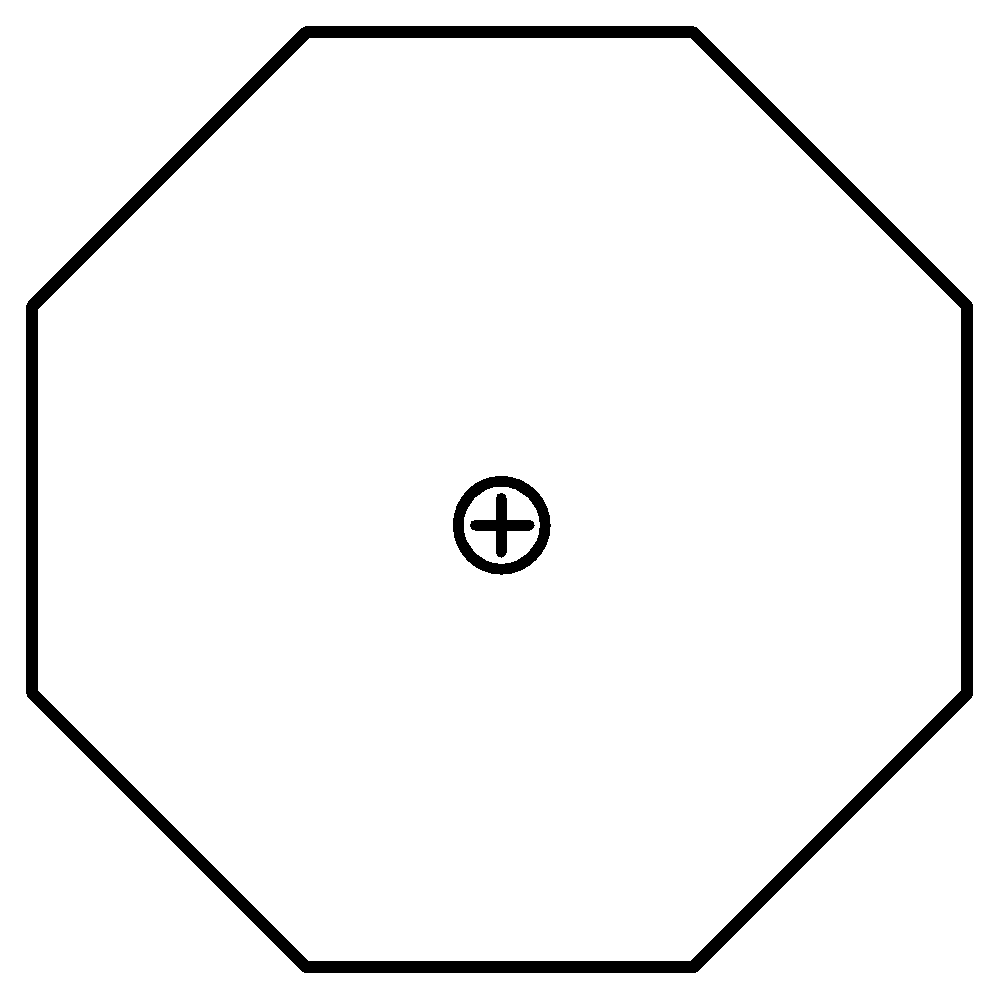
6 $\pi $ electrons
D.In Cyclopentadienyl cation, if a hydride anion \[\left( {H - } \right)\] is removed from the
\[sp3\] hybridized ring carbon, the cyclopentadienyl cation is formed, and hybridization of \[sp3\] ring carbon is altered to \[sp2\] .
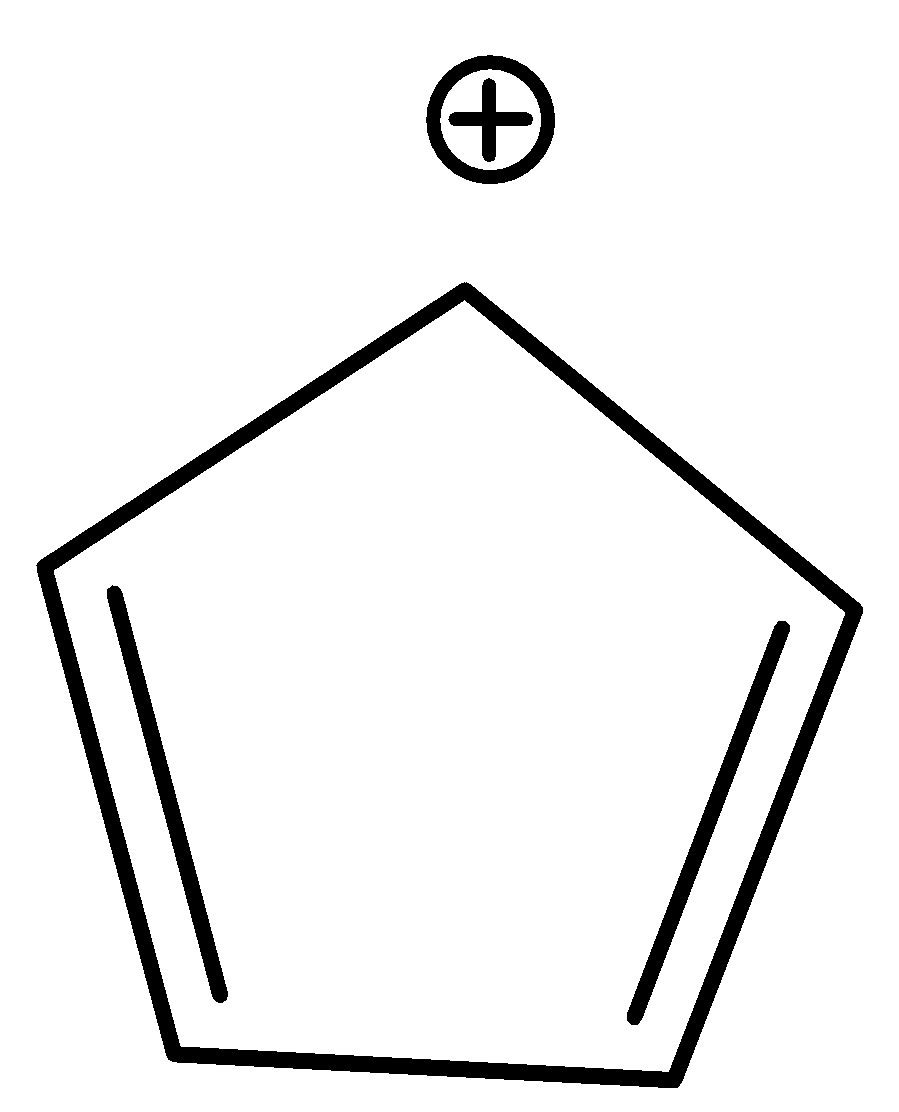
4 $\pi $ electrons
Thus, the cyclic cyclopentadienyl cation is planar and possesses a cyclic uninterrupted $\pi $ electron cloud. However, it does not follow Huckel’s rule as it has 4 $\pi $ electrons in a conjugated system. Thus, it is antiaromatic.
Hence option D is correct.
Note:
Molecules may change shape, becoming non-planar to avoid the instability of anti-aromaticity and therefore breaking some of the π interactions.
Complete step by step answer:
Non-aromatic does not follow Huckel’s rule. are highly unstable and highly reactive.
A.Benzene is cyclic, planar and has 6 $\pi $ electrons thus fulfilling Huckel’s rule. It is therefore aromatic.

6 $\pi $ electrons
B.Cyclooctatetraenyl Dianion has 10 $\pi $ electrons thus fulfilling Huckel’s rule. It is cyclic as well as planar. It is therefore aromatic.

10 $\pi $ electrons
C.Tropylium Ion has 6 $\pi $ electrons. Thus it also fulfils Huckel’s rule. It is cyclic as well as planar. Therefore, it is also aromatic.

6 $\pi $ electrons
D.In Cyclopentadienyl cation, if a hydride anion \[\left( {H - } \right)\] is removed from the
\[sp3\] hybridized ring carbon, the cyclopentadienyl cation is formed, and hybridization of \[sp3\] ring carbon is altered to \[sp2\] .

4 $\pi $ electrons
Thus, the cyclic cyclopentadienyl cation is planar and possesses a cyclic uninterrupted $\pi $ electron cloud. However, it does not follow Huckel’s rule as it has 4 $\pi $ electrons in a conjugated system. Thus, it is antiaromatic.
Hence option D is correct.
Note:
Molecules may change shape, becoming non-planar to avoid the instability of anti-aromaticity and therefore breaking some of the π interactions.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




