
Which of the following is not a monobasic acid?
Hydroiodic acid
Hypochlorous acid
Formic acid
Oxalic acid
Answer
586.2k+ views
Hint: By basicity of acid, we mean the number of hydronium ions formed by one molecule of acid in its aqueous solution. Monobasic acids are those which have only one hydrogen atom to donate.
Complete step by step answer:
In the case of the above question, option A is hydroiodic acid having chemical formula $HI$. When it is dissolved in an aqueous solution it gives only one ${H^ + }$ ion. Hence the basicity of hydroiodic acid is one so we can say it is monobasic.
$HI(aq) \to {H^ + } + {I^ - }$.
In the case of option B, the chemical formula of hypochlorous acid is $HClO$. It is a weak acid and is formed when chlorine dissolves in water. On dissociation it gives hypochlorite and one hydrogen ion. So again we can say it is also monobasic.
$HClO \to {H^ + } + Cl{O^ - }$
In the case of option C, the chemical formula for formic acid is $HCOOH$. It is the strongest fatty acid. On dissociation in aqueous solution, it also gives only one ${H^ + }$ion. So it is also monobasic.
In the case of option D, the chemical formula for oxalic acid is $HOOC - COOH$. When it dissociates it gives two ${H^ + }$ ions. So we can say it is not monobasic. it is dibasic due to two ionizable ${H^ + }$ ions.
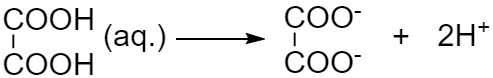
Hence the correct answer is option D .
Note:
There is no defined formula to find the basicity of acid and acidity of any base. Also, to find the acidity and basicity of a base and acid respectively, it is necessary to know what dissociation or association occurs in an aqueous solution of compound i.e. acid or base.
Complete step by step answer:
In the case of the above question, option A is hydroiodic acid having chemical formula $HI$. When it is dissolved in an aqueous solution it gives only one ${H^ + }$ ion. Hence the basicity of hydroiodic acid is one so we can say it is monobasic.
$HI(aq) \to {H^ + } + {I^ - }$.
In the case of option B, the chemical formula of hypochlorous acid is $HClO$. It is a weak acid and is formed when chlorine dissolves in water. On dissociation it gives hypochlorite and one hydrogen ion. So again we can say it is also monobasic.
$HClO \to {H^ + } + Cl{O^ - }$
In the case of option C, the chemical formula for formic acid is $HCOOH$. It is the strongest fatty acid. On dissociation in aqueous solution, it also gives only one ${H^ + }$ion. So it is also monobasic.
In the case of option D, the chemical formula for oxalic acid is $HOOC - COOH$. When it dissociates it gives two ${H^ + }$ ions. So we can say it is not monobasic. it is dibasic due to two ionizable ${H^ + }$ ions.

Hence the correct answer is option D .
Note:
There is no defined formula to find the basicity of acid and acidity of any base. Also, to find the acidity and basicity of a base and acid respectively, it is necessary to know what dissociation or association occurs in an aqueous solution of compound i.e. acid or base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




