
Which of the following is not a Meso compound?
A.
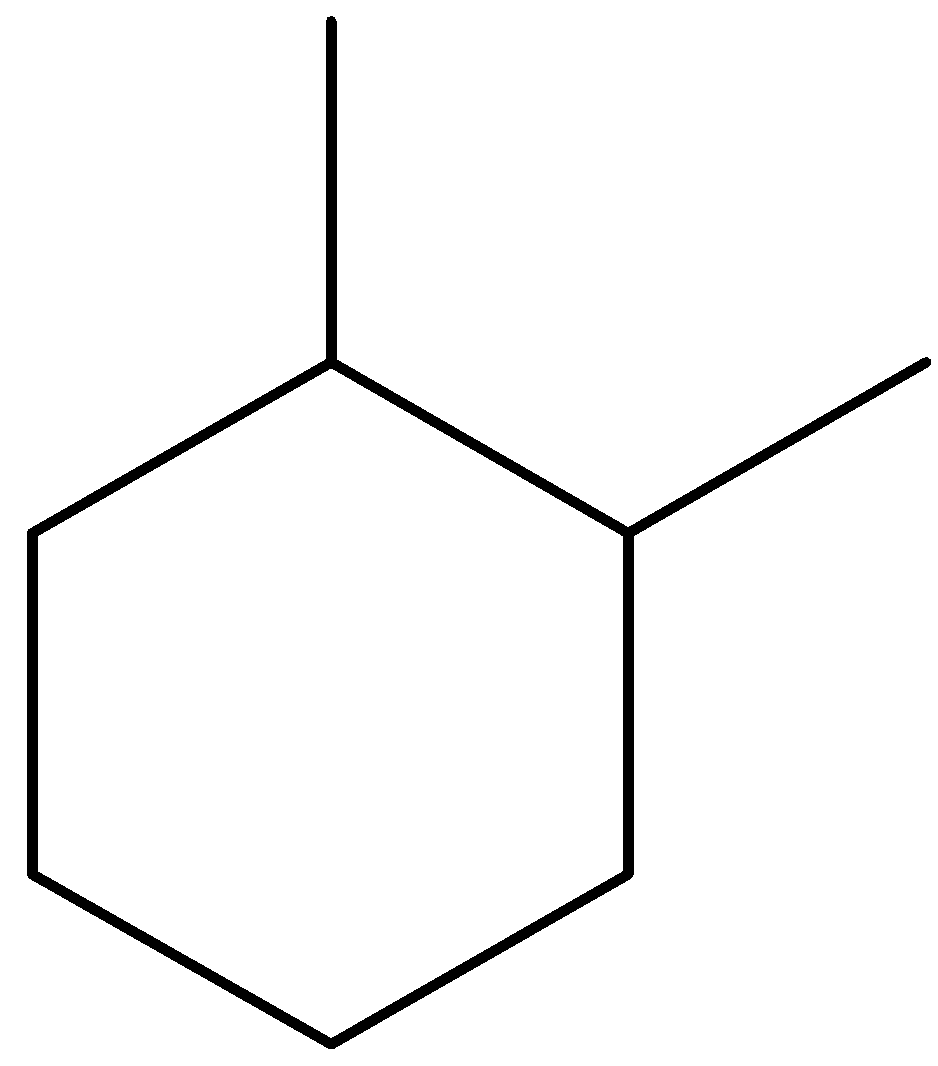
B.
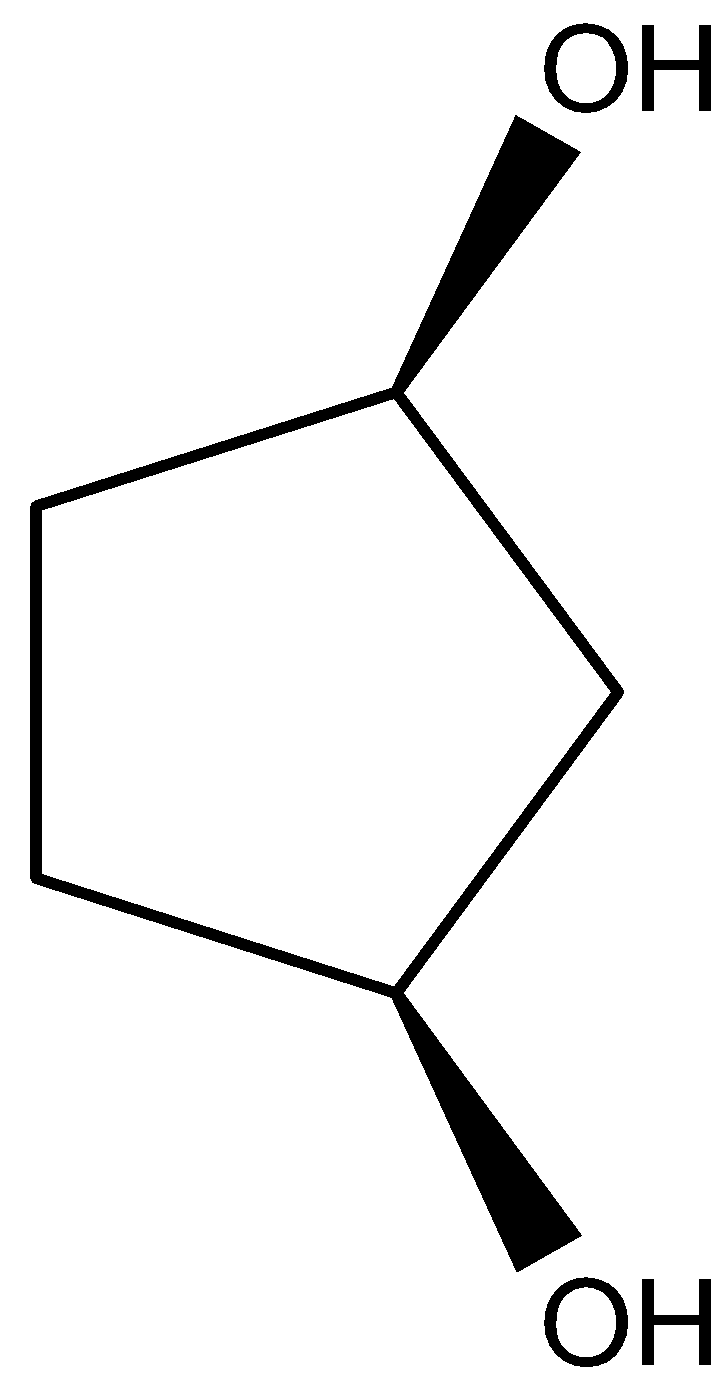
C.
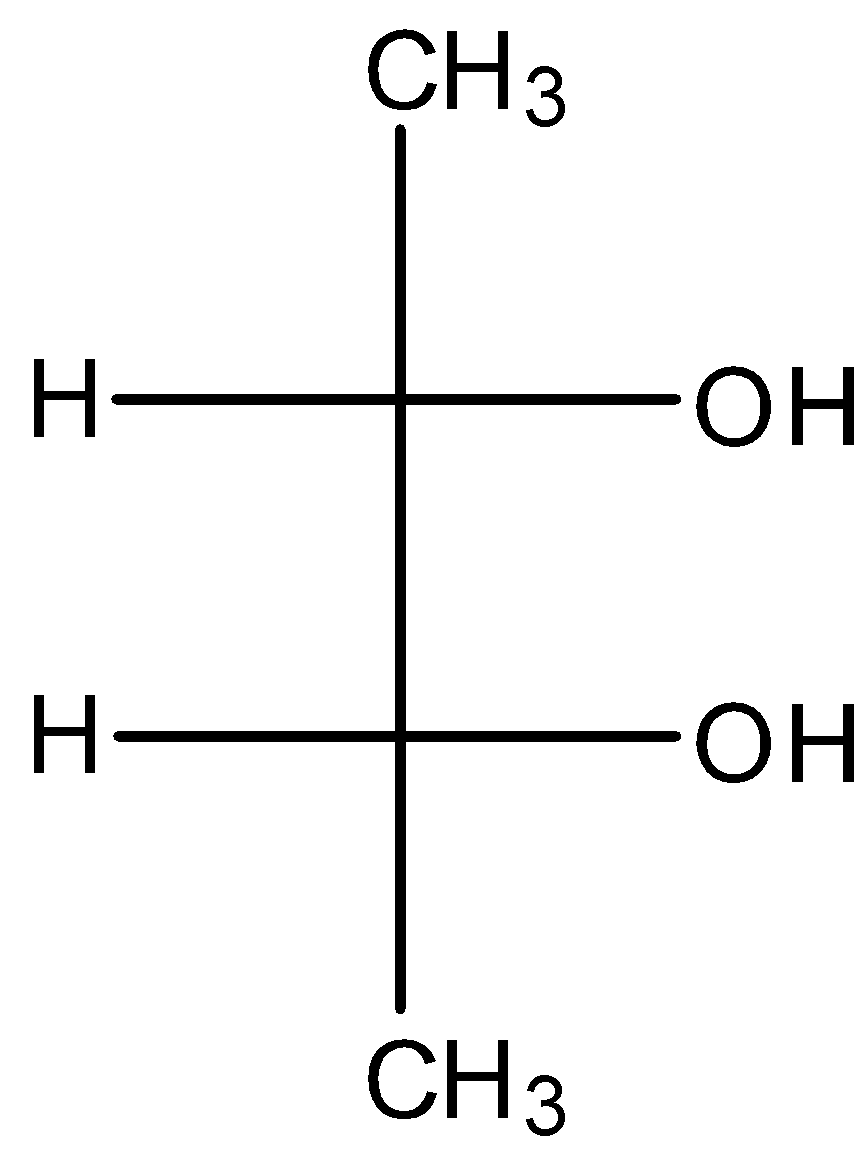
D.
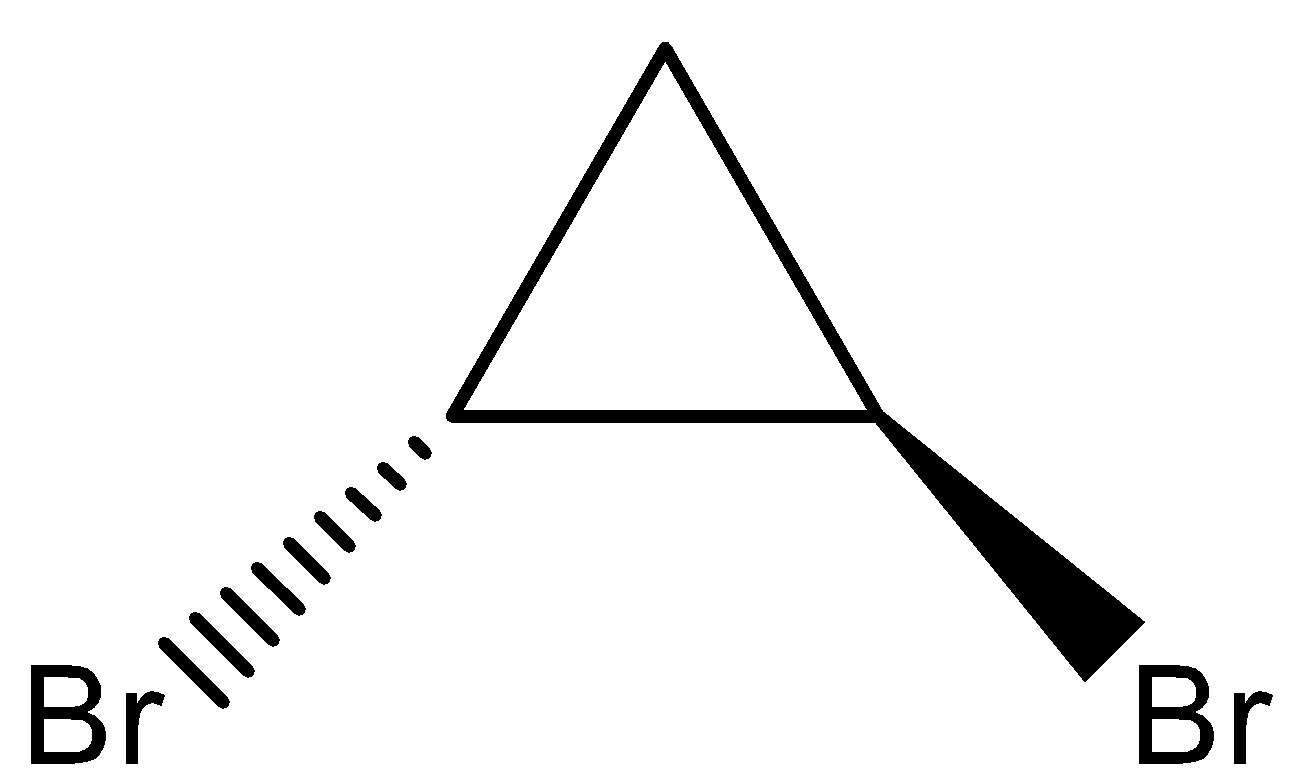




Answer
523.2k+ views
Hint: Meso isomers are stereoisomers which do not exhibit the optical active property. The meso isomers are achiral in nature but contain stereocenters and meso isomers are going to exhibit planes of symmetry and contain mirror images also.
Complete answer:
- In the question it is asked to find the compound which is not going to contain meso isomers among the given options.
- Means we have to find a compound which does not form meso isomers among the given options.
- Coming to the given options, Option A.
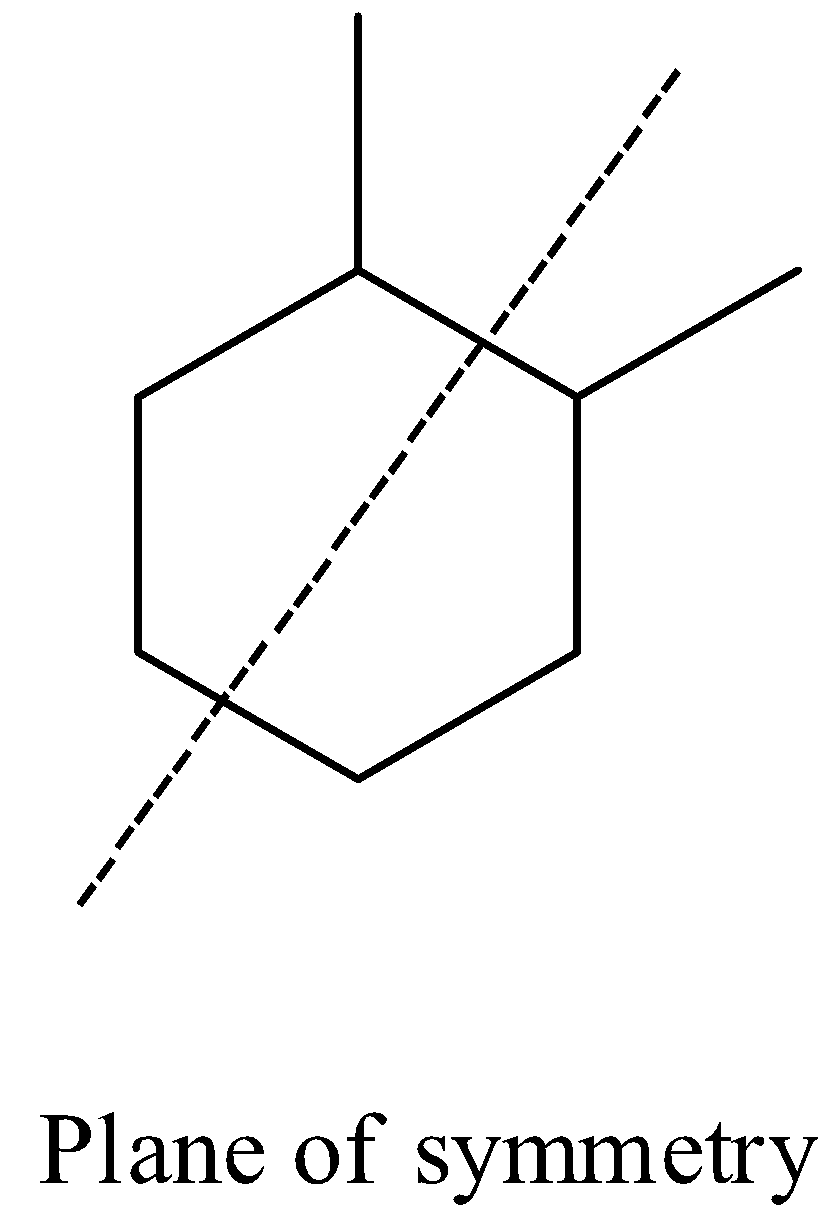
- The compound in option A contains two stereocenters and exhibits a plane of symmetry, then the compound in option A shows meso isomers.
- Coming to option B,
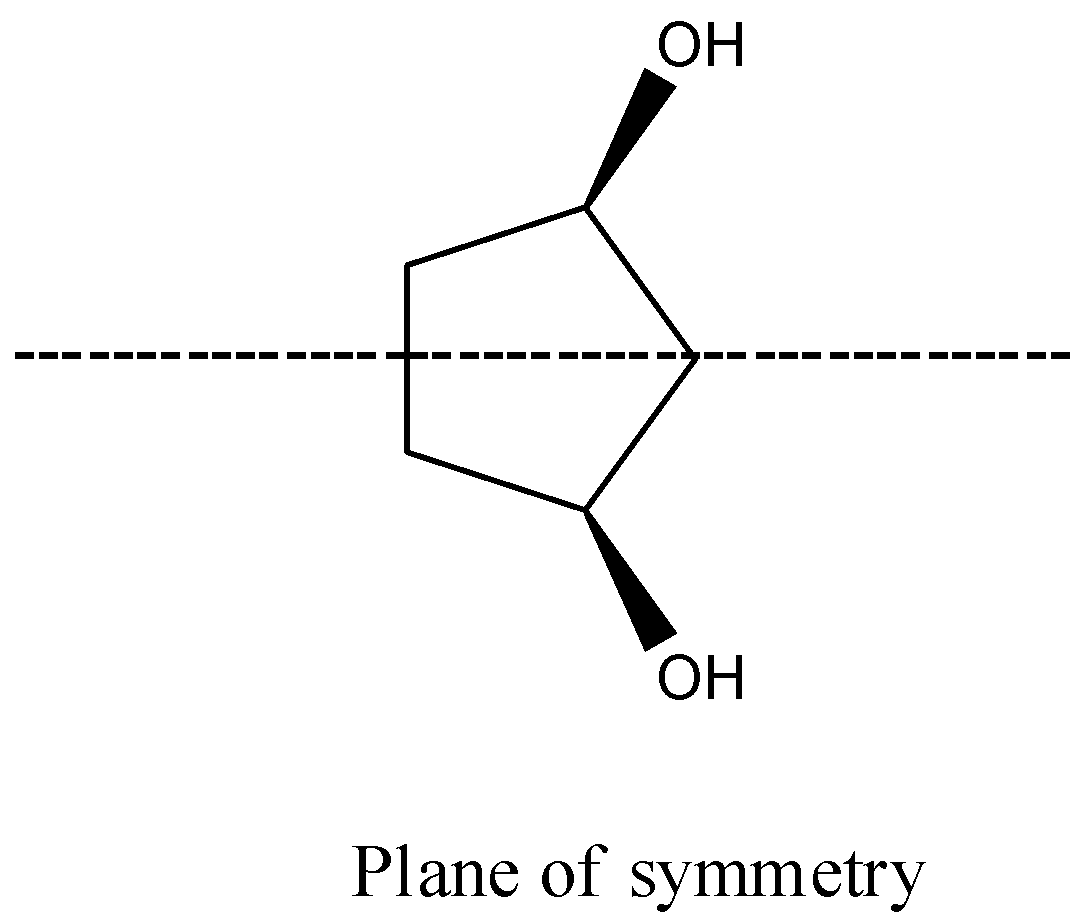
- The compound in option B contains two stereocenters and exhibits a plane of symmetry, then the compound in option B shows meso isomers.
- Coming to option C,
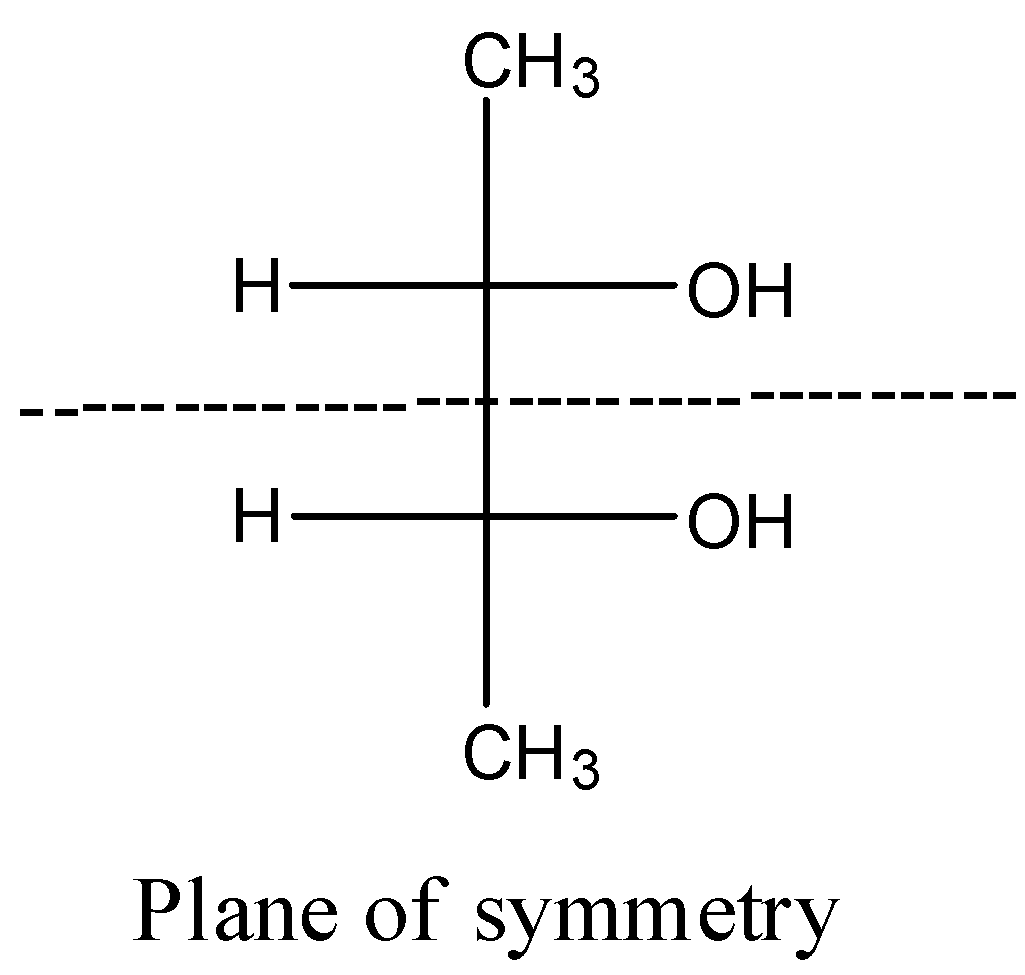
- The compound in option C contains two stereocenters and exhibits a plane of symmetry, then the compound in option C shows meso isomers.
- Coming to option D,
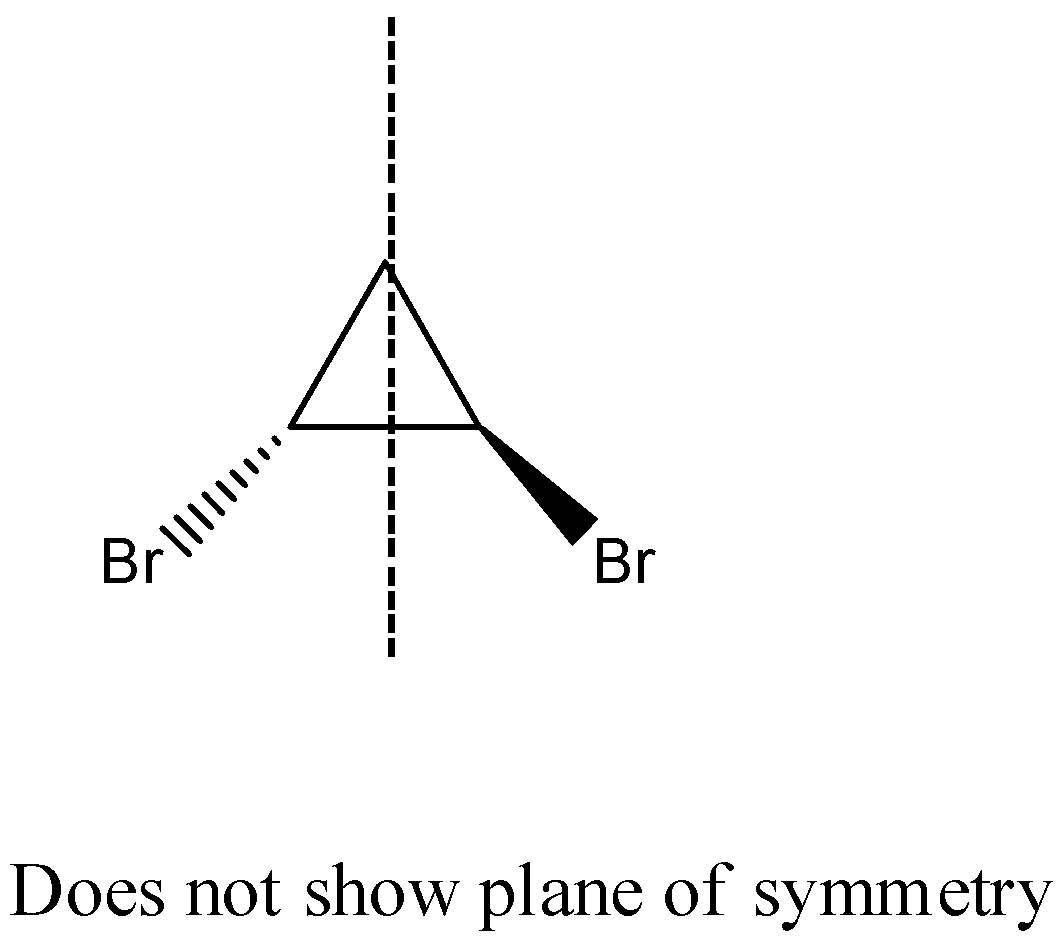
- The compound in the option D, does not show the plane of the symmetry.
Therefore the compound in the option D does not form meso isomers.
Note:
The compounds which have a plane of symmetry and stereocenters in it then the compound will form meso isomers. The plane of symmetry is a specific condition to identify the compound which exhibits meso isomers.
Complete answer:
- In the question it is asked to find the compound which is not going to contain meso isomers among the given options.
- Means we have to find a compound which does not form meso isomers among the given options.
- Coming to the given options, Option A.

- The compound in option A contains two stereocenters and exhibits a plane of symmetry, then the compound in option A shows meso isomers.
- Coming to option B,

- The compound in option B contains two stereocenters and exhibits a plane of symmetry, then the compound in option B shows meso isomers.
- Coming to option C,

- The compound in option C contains two stereocenters and exhibits a plane of symmetry, then the compound in option C shows meso isomers.
- Coming to option D,

- The compound in the option D, does not show the plane of the symmetry.
Therefore the compound in the option D does not form meso isomers.
Note:
The compounds which have a plane of symmetry and stereocenters in it then the compound will form meso isomers. The plane of symmetry is a specific condition to identify the compound which exhibits meso isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




