
Which of the following is not a Lewis acid?
(a)- $FeC{{l}_{3}}$
(b)- $BC{{l}_{3}}$
(c)- $ZnC{{l}_{2}}$
(d)- $N{{H}_{3}}$
Answer
585k+ views
Hint: Lewis explained that an acid accepts a pair of electrons and the base donates a pair of electrons. So, we can identify the compound which has electrons to donate is not a Lewis acid.
Complete step by step answer:
According to Lewis, an acid can be defined as a substance that can accept a pair of the electron, and a base is that substance that can donate a pair of electrons.
So, the acid must be an electron-deficient compound and the base must be an electron-rich compound.
In $FeC{{l}_{3}}$, the iron atom has an incomplete octet. The iron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.
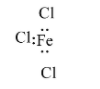
In $BC{{l}_{3}}$, the boron atom has an incomplete octet. The boron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.

In $ZnC{{l}_{2}}$, the zinc atom has an incomplete octet. The zinc atom is bonded with two chlorine atoms, this means that zinc is in +2 oxidation state, hence it can accept a pair of electrons. Therefore, it is a Lewis acid.

In $N{{H}_{3}}$, the nitrogen atom is bonded with three hydrogen atoms, but the valence electrons in nitrogen are 5. So it has a pair of electrons that are non-bonded. So, it donates a pair of electrons. It is a Lewis base.
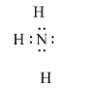
So, the correct answer is an option (d)- $N{{H}_{3}}$
Note: Some other examples of Lewis acid in molecular form are $B{{F}_{3}}$. Cations that acts as Lewis acid are $A{{g}^{+}},C{{u}^{2+}},F{{e}^{3+}}$, etc. The molecules having empty d-orbital also act as Lewis acid, $Si{{F}_{4}}$.
Complete step by step answer:
According to Lewis, an acid can be defined as a substance that can accept a pair of the electron, and a base is that substance that can donate a pair of electrons.
So, the acid must be an electron-deficient compound and the base must be an electron-rich compound.
In $FeC{{l}_{3}}$, the iron atom has an incomplete octet. The iron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.
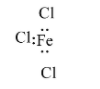
In $BC{{l}_{3}}$, the boron atom has an incomplete octet. The boron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.

In $ZnC{{l}_{2}}$, the zinc atom has an incomplete octet. The zinc atom is bonded with two chlorine atoms, this means that zinc is in +2 oxidation state, hence it can accept a pair of electrons. Therefore, it is a Lewis acid.

In $N{{H}_{3}}$, the nitrogen atom is bonded with three hydrogen atoms, but the valence electrons in nitrogen are 5. So it has a pair of electrons that are non-bonded. So, it donates a pair of electrons. It is a Lewis base.
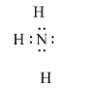
So, the correct answer is an option (d)- $N{{H}_{3}}$
Note: Some other examples of Lewis acid in molecular form are $B{{F}_{3}}$. Cations that acts as Lewis acid are $A{{g}^{+}},C{{u}^{2+}},F{{e}^{3+}}$, etc. The molecules having empty d-orbital also act as Lewis acid, $Si{{F}_{4}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




