
Which of the following is most stable:
A.
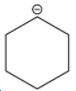
B.
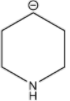
C.
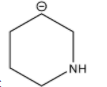
D.
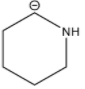




Answer
574.2k+ views
Hint:Stability of the carbanion depends upon the delocalization of the negative charge. The higher the delocalization of the negative charge, the higher will be the stability of the carbanion. The stability of the carbanion is also dependent upon the presence of an electron withdrawing group, higher the number of electron withdrawing groups higher will be the stability of the carbanion and vice-versa. For example the stability order of carbanion is as follows.
Complete step by step answer:
Among the given structures of the carbanion. The presence of the amine group stabilizes the negative charge. In this case, nitrogen is an electronegative element that shows an electron-withdrawing effect. Due to the electron-withdrawing nature of the nitrogen, the carbanion gets stability.
Now inductive effect depends upon the distance. With increasing the distance between the amine group and negative charge, the stability of the carbanion decreases and vice-versa.
Therefore, based on the inductive effect the stability is highest for option D. As the amine group is in the proximity which stabilizes the carbanion.
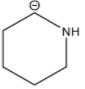
So, the correct option is D.
Note:
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. It is defined as any even-electron cation that possesses a significant positive charge on the carbon atom and the alpha carbon refers to the first carbon atom that attaches to a functional group, such as a carbonyl.
Complete step by step answer:
Among the given structures of the carbanion. The presence of the amine group stabilizes the negative charge. In this case, nitrogen is an electronegative element that shows an electron-withdrawing effect. Due to the electron-withdrawing nature of the nitrogen, the carbanion gets stability.
Now inductive effect depends upon the distance. With increasing the distance between the amine group and negative charge, the stability of the carbanion decreases and vice-versa.
Therefore, based on the inductive effect the stability is highest for option D. As the amine group is in the proximity which stabilizes the carbanion.

So, the correct option is D.
Note:
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. It is defined as any even-electron cation that possesses a significant positive charge on the carbon atom and the alpha carbon refers to the first carbon atom that attaches to a functional group, such as a carbonyl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




