
Which of the following is most likely to undergo a favorable hydride shift?
A.

B.
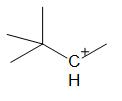
C.
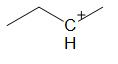
D.





Answer
579k+ views
Hint: A carbocation molecule undergoes a hydride shift in order to obtain a more stable carbocation, if possible. Tertiary carbocations are more stable than secondary carbocations which are more stable than primary carbocations.
Complete step by step answer:
We shall analyze each of the options and the stability of the products formed by hydride shift,
For option A:
The hydride shift shall take place from the tertiary carbon atom resulting into the formation of a tertiary carbocation thus providing extra stability. Furthermore, the carbocation formed after a hydride shift tends to rearrange again and form a cyclopentane carbocation.
We know that cyclopentane is more stable than cyclobutane due to an increased bond angle and reduced strain on the ring. Thus we can say that this compound is favorable to undergo a hydride shift.
For option B:
The two adjacent carbon atoms of the positively charged carbon atom are a primary and tertiary group. The formation of a tertiary carbocation is favored. But the rearrangement is obtained through a methide shift rather than a hydride shift since the tertiary carbon atom is not bonded to hydrogen atoms.
Thus, hydride shift does not occur in the following compound.
For option C:
Option C has a symmetrical molecule. A more stable carbocation than the already existing one cannot be formed through rearrangement.
Thus, this compound does not undergo hydride shift.
For option D:
The compound in option D undergoes ring expansion to form a more stable 5 membered ring. But the ring expands by the breaking of a bond and not by hydride shift.
Thus, we can conclude that the correct option is A.
Note:
Hydride shift involves the movement of a two electron hydrogen from the unimolecular substitution over to the neighboring carbon. The phenomenon of hydride shift is generally seen in the reaction of alcohol and hydrogen halides.
Complete step by step answer:
We shall analyze each of the options and the stability of the products formed by hydride shift,
For option A:
The hydride shift shall take place from the tertiary carbon atom resulting into the formation of a tertiary carbocation thus providing extra stability. Furthermore, the carbocation formed after a hydride shift tends to rearrange again and form a cyclopentane carbocation.
We know that cyclopentane is more stable than cyclobutane due to an increased bond angle and reduced strain on the ring. Thus we can say that this compound is favorable to undergo a hydride shift.
For option B:
The two adjacent carbon atoms of the positively charged carbon atom are a primary and tertiary group. The formation of a tertiary carbocation is favored. But the rearrangement is obtained through a methide shift rather than a hydride shift since the tertiary carbon atom is not bonded to hydrogen atoms.
Thus, hydride shift does not occur in the following compound.
For option C:
Option C has a symmetrical molecule. A more stable carbocation than the already existing one cannot be formed through rearrangement.
Thus, this compound does not undergo hydride shift.
For option D:
The compound in option D undergoes ring expansion to form a more stable 5 membered ring. But the ring expands by the breaking of a bond and not by hydride shift.
Thus, we can conclude that the correct option is A.
Note:
Hydride shift involves the movement of a two electron hydrogen from the unimolecular substitution over to the neighboring carbon. The phenomenon of hydride shift is generally seen in the reaction of alcohol and hydrogen halides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




