
Which of the following is most acidic?
A.
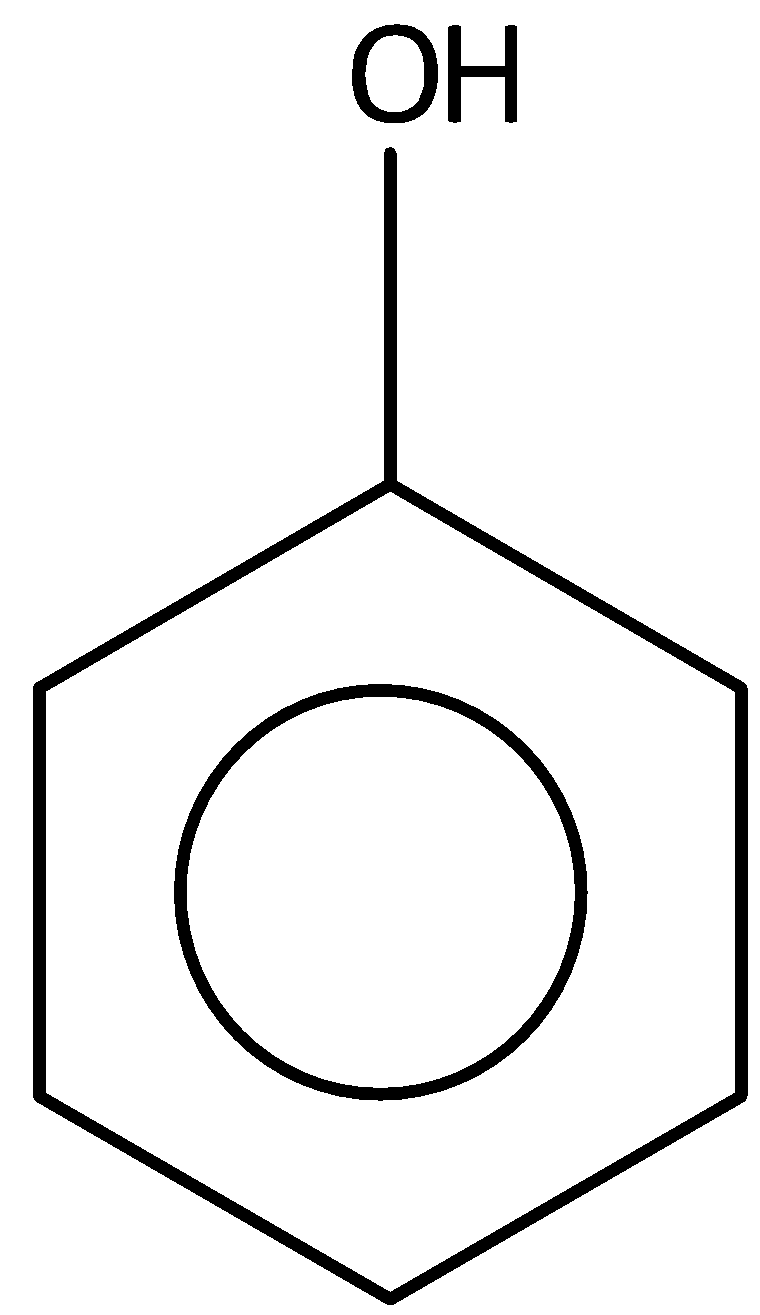
B.
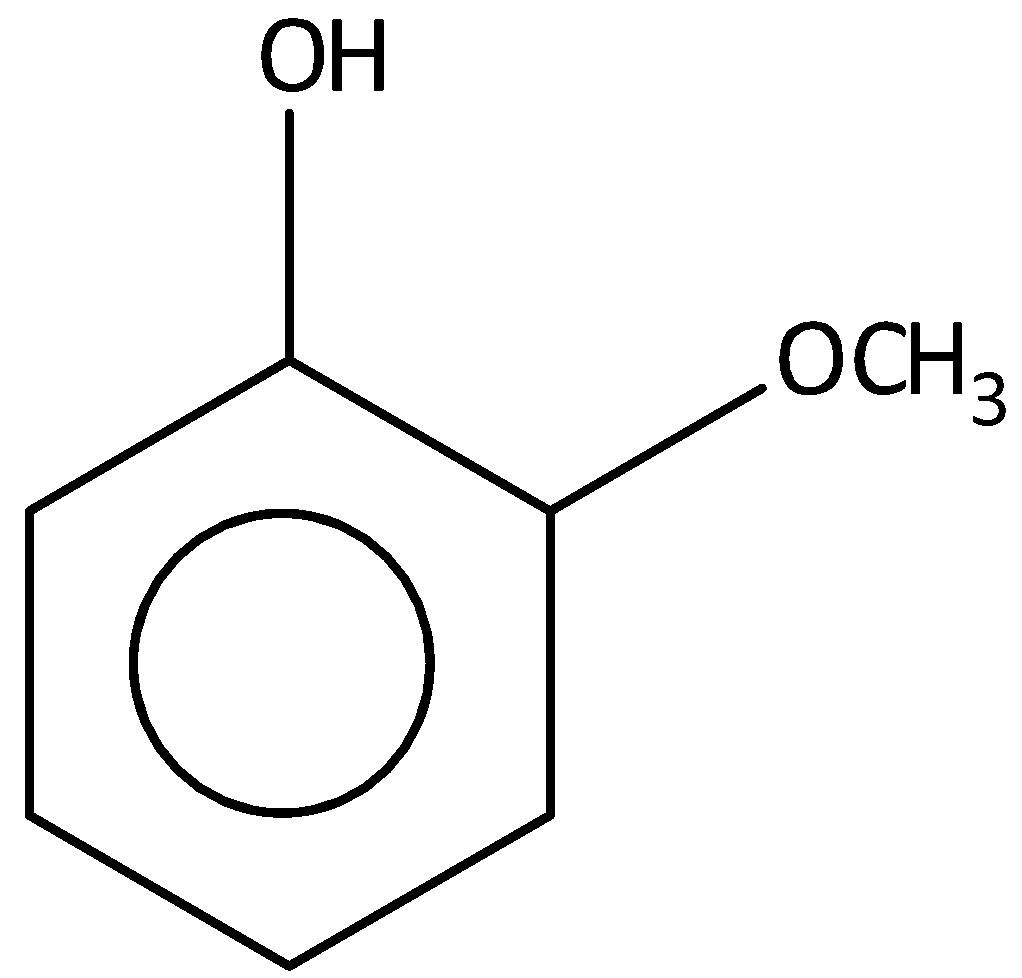
C.
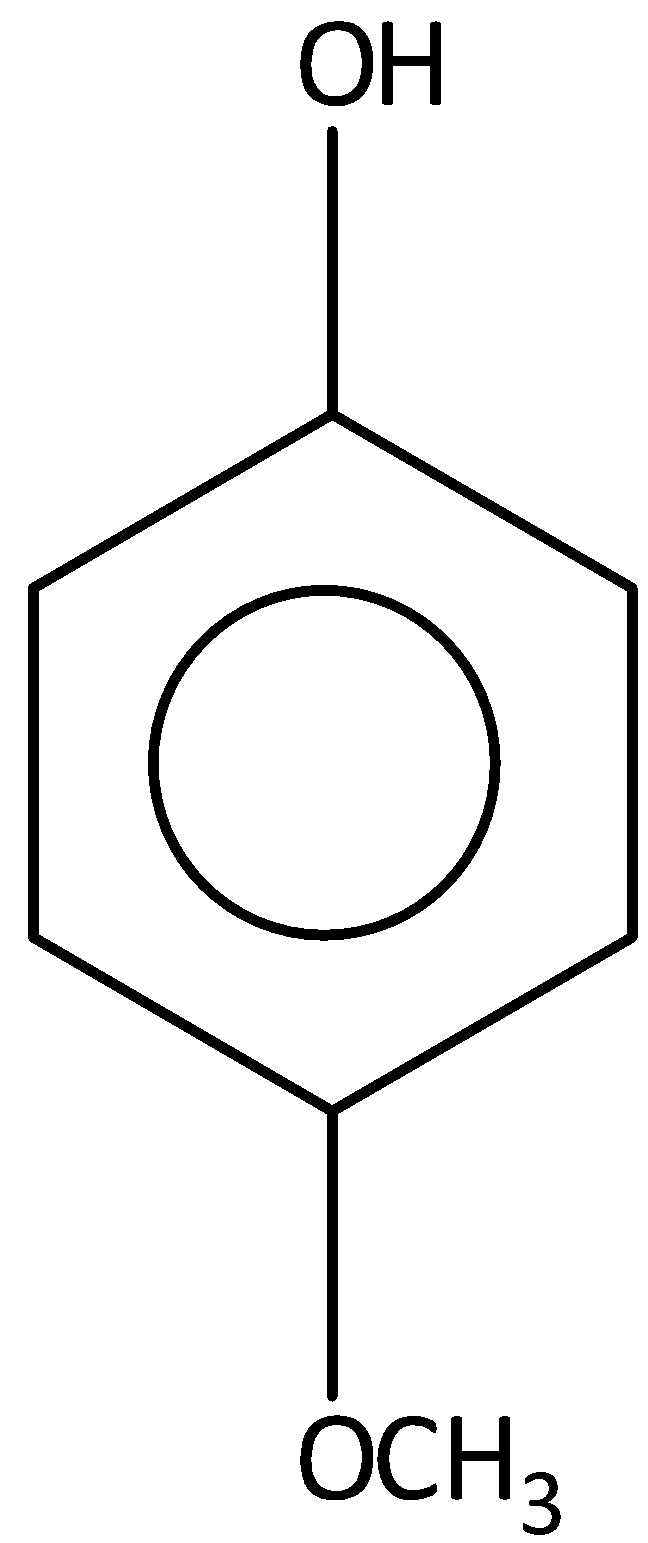
D.
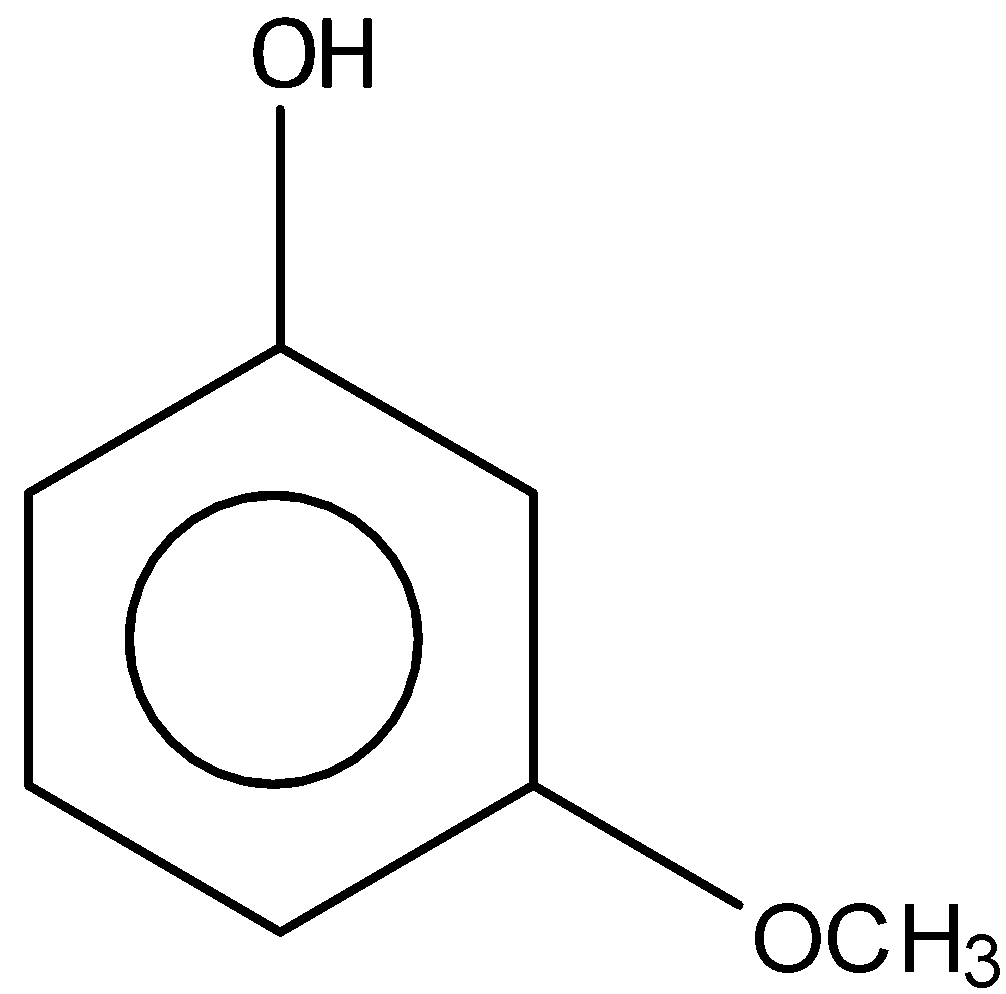




Answer
495.6k+ views
Hint: As we know that the acids and bases are the chemical compounds. When they react together, there is a formation of salt and water. The acid is a chemical substance which contains hydrogen and it donates the protons to another substance. And the base is a chemical substance which accepts the hydrogen ion. The acidic substances are sour in taste and it turns blue litmus to red. The base has a bitter taste and it turns red litmus to blue.
Complete answer:
The phenol is not most acidic from the given compound. Hence, option (A) is incorrect.
The \[2 - \] methoxy phenol is not most acidic from the given compounds. Hence, option (B) is incorrect.
Among the given compounds \[4 - \] methoxy phenol is not the most acidic compound. Hence, option (C) is incorrect.
Among the given options, \[3 - \] methoxy phenol is the most acidic compound. Because, the methoxy group is inductive withdrawing and they are mesomeric donating. We know that the mesmeric effect will override the inductive effect. But the mesomeric effect is only valid when the substituent is attached on the para position and the ortho position.
And the addition of an electron donating group to the phenol ring will affect the acidity. Because, the acidity will decrease when an electron donating group is attached to the phenol ring. But the acidity will increase when the electron withdrawing group is attached to the phenol ring. Thus, we can say that the meta- methoxy phenol is more acidic than the others.
Hence, option (D) is correct.
Note:
We need to know that the acidity of the compound increases when an electron withdrawing group is present in that compound. The inductive effect will affect the acidity and basicity of a compound. Because, when the number of negative inductive groups is increasing, there is a chance to increase the acidity. And if the positive inductive group is present, there is a chance to increase the basicity.
Complete answer:
The phenol is not most acidic from the given compound. Hence, option (A) is incorrect.
The \[2 - \] methoxy phenol is not most acidic from the given compounds. Hence, option (B) is incorrect.
Among the given compounds \[4 - \] methoxy phenol is not the most acidic compound. Hence, option (C) is incorrect.
Among the given options, \[3 - \] methoxy phenol is the most acidic compound. Because, the methoxy group is inductive withdrawing and they are mesomeric donating. We know that the mesmeric effect will override the inductive effect. But the mesomeric effect is only valid when the substituent is attached on the para position and the ortho position.
And the addition of an electron donating group to the phenol ring will affect the acidity. Because, the acidity will decrease when an electron donating group is attached to the phenol ring. But the acidity will increase when the electron withdrawing group is attached to the phenol ring. Thus, we can say that the meta- methoxy phenol is more acidic than the others.
Hence, option (D) is correct.
Note:
We need to know that the acidity of the compound increases when an electron withdrawing group is present in that compound. The inductive effect will affect the acidity and basicity of a compound. Because, when the number of negative inductive groups is increasing, there is a chance to increase the acidity. And if the positive inductive group is present, there is a chance to increase the basicity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




