
Which of the following is least stable:-
(A) $C{H_3} - \mathop {CH}\limits^ + - C{H_3}$
(B) $C{H_3} - C{H_2} - \mathop {C{H_2}}\limits^ + $
(C)
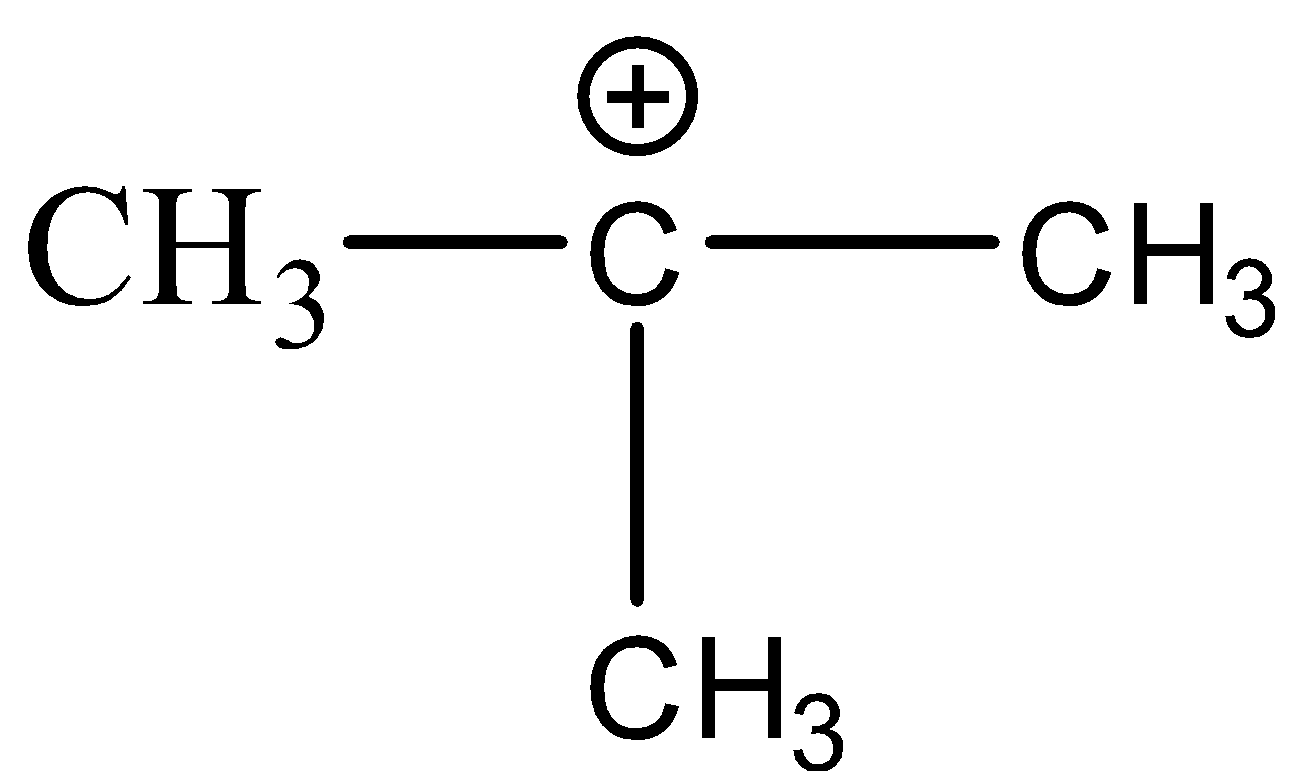
(D)
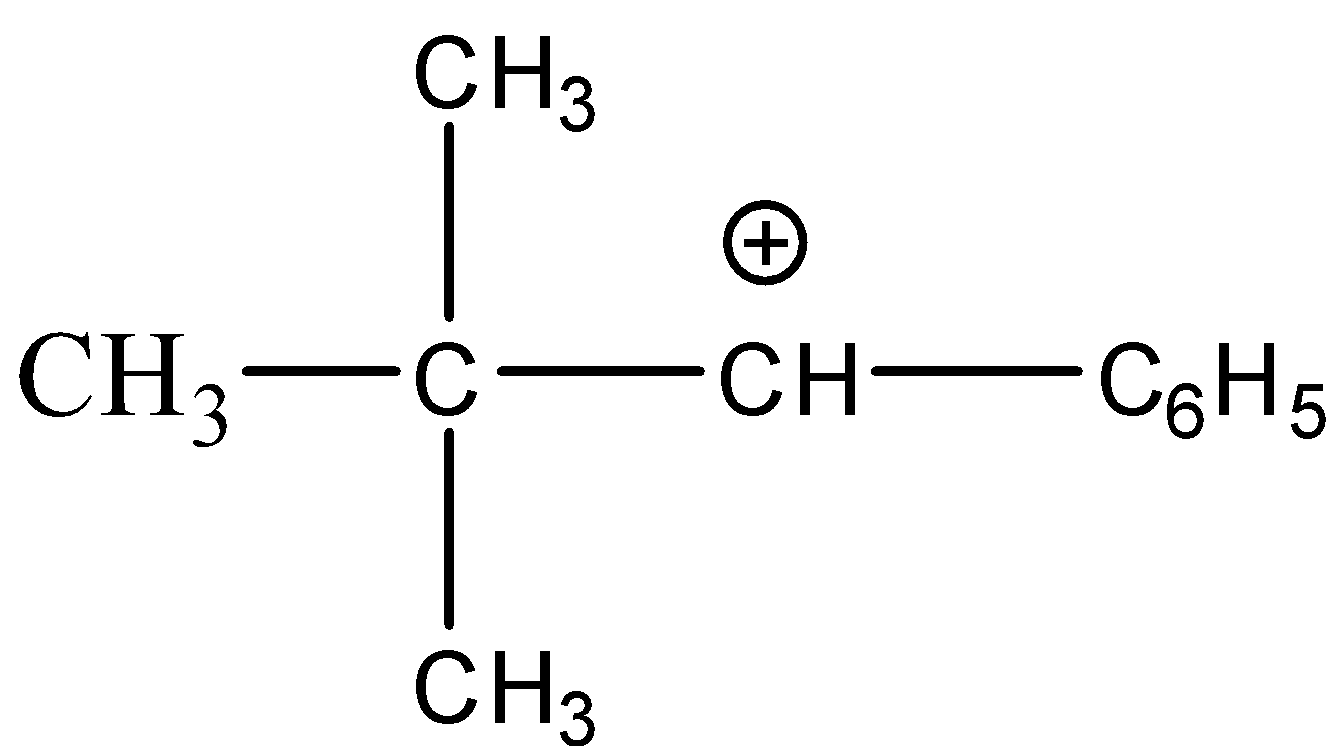


Answer
546.6k+ views
Hint:In order to find out which species is least stable we have to compare the stability order of these given carbocations. A carbocation is a species that contains a carbon atom with a positive charge and three bonds.
Complete step by step answer:
-Earlier, Carbocations were called carbonium ions. Now they are defined as any even-electron cation that possesses a particular positive charge on the carbon atom The carbon atom of the carbocation has $s{p^2}$ hybridization and trigonal planar geometry.
-As carbocations are electron-deficient species due to incomplete octet, they are highly unstable and therefore reactive in nature. Hence, they are also called electrophiles.
-Based on the number of carbon groups that are bonded to the carbon, the carbocation can are divided into $4$ types. These are methyl, primary, secondary or tertiary carbocation.
-In methyl carbocation, no carbon is bonded to the carbon-containing positive charge. In the case of primary, secondary, and tertiary carbocations the number of carbon groups attached to the carbon with the positive charge are $1,2$ and $3$ respectively. They are also known as ${1^0},{2^0}$ and ${3^0}$ carbocation.
-The stability of these carbocations depends upon the hyper conjugative structures i.e. more the number of substituents bonded to the carbocation more will be the stability of the carbocation. Hence, the order of stability of carbocation is given as;
$\mathop {C{H_3}}\limits^ + > {1^0}carbocation > {2^0}carbocation > {3^0}carbocation$
-Hence, out of the given options, the carbocation that has the least number of substituents is $C{H_3} - C{H_2} - \mathop {C{H_2}}\limits^ + $. So, it will be the least stable species.
Therefore, Option (B) is correct.
Note:
As $C{H_3}$ groups are electron-donating groups, substitution helps in decreasing the electron poverty of the carbocation atom. Hence Substituted carbocations are more stable than less substituted carbocations.
Complete step by step answer:
-Earlier, Carbocations were called carbonium ions. Now they are defined as any even-electron cation that possesses a particular positive charge on the carbon atom The carbon atom of the carbocation has $s{p^2}$ hybridization and trigonal planar geometry.
-As carbocations are electron-deficient species due to incomplete octet, they are highly unstable and therefore reactive in nature. Hence, they are also called electrophiles.
-Based on the number of carbon groups that are bonded to the carbon, the carbocation can are divided into $4$ types. These are methyl, primary, secondary or tertiary carbocation.
-In methyl carbocation, no carbon is bonded to the carbon-containing positive charge. In the case of primary, secondary, and tertiary carbocations the number of carbon groups attached to the carbon with the positive charge are $1,2$ and $3$ respectively. They are also known as ${1^0},{2^0}$ and ${3^0}$ carbocation.
-The stability of these carbocations depends upon the hyper conjugative structures i.e. more the number of substituents bonded to the carbocation more will be the stability of the carbocation. Hence, the order of stability of carbocation is given as;
$\mathop {C{H_3}}\limits^ + > {1^0}carbocation > {2^0}carbocation > {3^0}carbocation$
-Hence, out of the given options, the carbocation that has the least number of substituents is $C{H_3} - C{H_2} - \mathop {C{H_2}}\limits^ + $. So, it will be the least stable species.
Therefore, Option (B) is correct.
Note:
As $C{H_3}$ groups are electron-donating groups, substitution helps in decreasing the electron poverty of the carbocation atom. Hence Substituted carbocations are more stable than less substituted carbocations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




