
Which of the following is least polar and most stable conformers?
A)
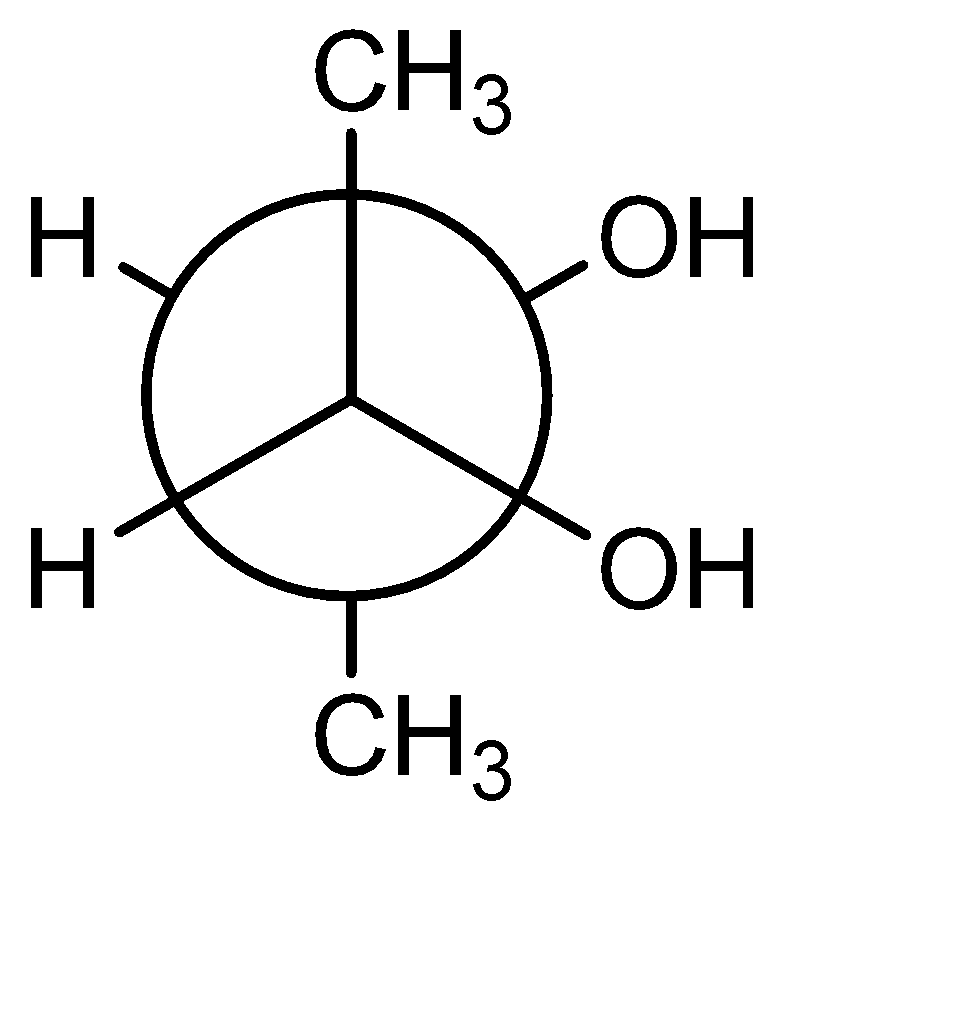
B)
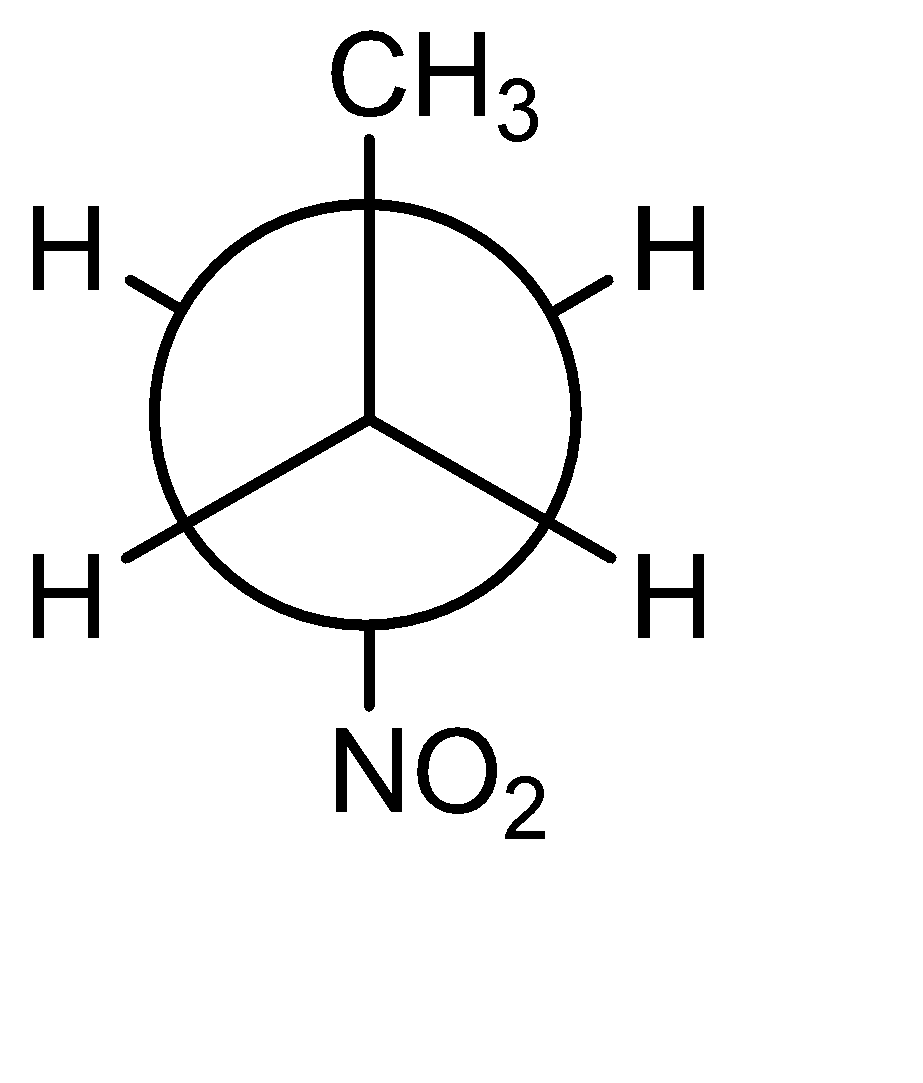
C)

D)





Answer
480.6k+ views
Hint: The question can be solved by knowing the stability of the various conformers of the Newman Projection. If any one conformer is considered it is called a conformational isomer. The most stable conformer will be the one with the least repulsion and hindrance. The polarity will depend on the electronegativity of the substituents.
Complete answer:
The various arrangements obtained by the rotation of the sigma bond are known as conformations The Newman projection is the most convenient way to visualize different conformations of a molecule. In this projection, we look lengthwise down a specific bond of interest. The front carbon atom is depicted by a dot and that of the back carbon by a circle.
Note that the bond formed between the C-H bond of the front carbon and C-H bond of the back carbon is known as dihedral angle. The most stable and lowest energy conformer is known as ‘Staggered’ where the dihedral angles are $ {60^ \circ } $ and the distance between front and back C-H is maximised. It is also known as the ‘Anti’ conformation.
The most unstable or highest energy conformer is known as ‘Eclipsed’ where the dihedral angle is $ {0^ \circ } $ . In the given options B, C and D are purely staggered conformations. The effect of substituents on the stability can be explained by the ‘A’ value. The more the A value, the more bulky the group is considered. The two bulky groups should be at the maximum distance to avoid repulsion (i.e. in Anti conformation). The A values of Methyl, Bromo, Fluro, Nitro and Chloro are: 1.7, 0.38,0.43, 1.1 respectively. The A value is the Highest for methyl, means the Option A should be the most stable, but methyl group is least polar, hence incorrect.
Option C has Br and F as substituents at staggered position, making it the most stable and the molecule will be the most polar.
Hence the correct answer is Option (C).
Note:
We obtained the Gauche conformation if we rotate the molecule by $ {60^ \circ } $ at an eclipsed position. Further a rotation of $ {60^ \circ } $ to gauche will give us eclipsed conformation again. Another rotation by $ {60^ \circ } $ will give us anti. The energy of various conformers can be given by the graph below:
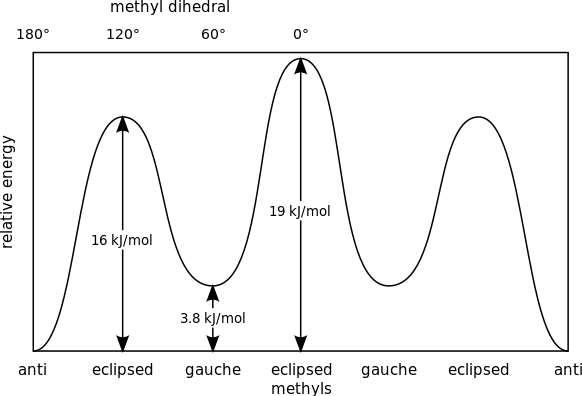
Complete answer:
The various arrangements obtained by the rotation of the sigma bond are known as conformations The Newman projection is the most convenient way to visualize different conformations of a molecule. In this projection, we look lengthwise down a specific bond of interest. The front carbon atom is depicted by a dot and that of the back carbon by a circle.
Note that the bond formed between the C-H bond of the front carbon and C-H bond of the back carbon is known as dihedral angle. The most stable and lowest energy conformer is known as ‘Staggered’ where the dihedral angles are $ {60^ \circ } $ and the distance between front and back C-H is maximised. It is also known as the ‘Anti’ conformation.
The most unstable or highest energy conformer is known as ‘Eclipsed’ where the dihedral angle is $ {0^ \circ } $ . In the given options B, C and D are purely staggered conformations. The effect of substituents on the stability can be explained by the ‘A’ value. The more the A value, the more bulky the group is considered. The two bulky groups should be at the maximum distance to avoid repulsion (i.e. in Anti conformation). The A values of Methyl, Bromo, Fluro, Nitro and Chloro are: 1.7, 0.38,0.43, 1.1 respectively. The A value is the Highest for methyl, means the Option A should be the most stable, but methyl group is least polar, hence incorrect.
Option C has Br and F as substituents at staggered position, making it the most stable and the molecule will be the most polar.
Hence the correct answer is Option (C).
Note:
We obtained the Gauche conformation if we rotate the molecule by $ {60^ \circ } $ at an eclipsed position. Further a rotation of $ {60^ \circ } $ to gauche will give us eclipsed conformation again. Another rotation by $ {60^ \circ } $ will give us anti. The energy of various conformers can be given by the graph below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




