
Which of the following is incorrect about a heterogeneous mixture?
A. Constituents of heterogeneous mixtures are uniformly mixed.
B. It is composed of multiple separate parts.
C. It is composed of a chemical solution.
D. All of these
Answer
508.2k+ views
Hint: The physical combination of two or more substances without losing the identity of each substance is known as a mixture. A mixture can be a form of solution, suspensions and colloids. The substances in a mixture do not undergo a chemical change and it is not necessary to mix each element in a specific ratio to form a mixture.
Complete answer:
There are two primary types of mixture which are discussed below:
Homogeneous mixture: These are the type of mixtures in which the substances or components are mixed uniformly and distributed in a uniform composition throughout the mixture. Only one phase of matter is observed in the homogeneous mixtures. The homogeneous mixtures are also known as solutions.
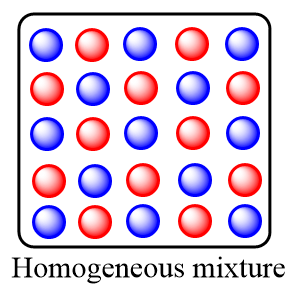
Heterogeneous mixture: These are the type of mixtures in which the substances or components are completely mixed and are distributed non uniformly within the mixture. It consists of a non-uniform composition and there exists two or more phases of solution within the heterogeneous mixture.
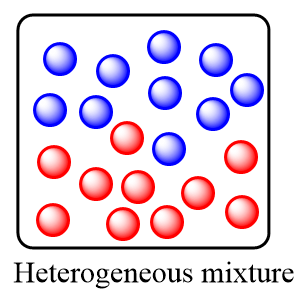
The key-points for a heterogeneous mixture are as follows:
1.Particles or components are distributed non uniformly.
2.It is composed of multiple separate parts i.e.; it consists of two or more phases of matter within the mixture.
3.Components of the mixture can be separated out physically i.e., it is not composed of a chemical solution.
Therefore, options (A) and (C) are the correct answers.
Note:
Remember that in homogeneous mixture, as the distribution of the components is uniform, so we cannot judge a homogeneous mixture by just looking at it whereas in a heterogeneous mixture, due to the non-uniform composition of substance, one can easily identify it by just looking at the mixture.
Complete answer:
There are two primary types of mixture which are discussed below:
Homogeneous mixture: These are the type of mixtures in which the substances or components are mixed uniformly and distributed in a uniform composition throughout the mixture. Only one phase of matter is observed in the homogeneous mixtures. The homogeneous mixtures are also known as solutions.

Heterogeneous mixture: These are the type of mixtures in which the substances or components are completely mixed and are distributed non uniformly within the mixture. It consists of a non-uniform composition and there exists two or more phases of solution within the heterogeneous mixture.

The key-points for a heterogeneous mixture are as follows:
1.Particles or components are distributed non uniformly.
2.It is composed of multiple separate parts i.e.; it consists of two or more phases of matter within the mixture.
3.Components of the mixture can be separated out physically i.e., it is not composed of a chemical solution.
Therefore, options (A) and (C) are the correct answers.
Note:
Remember that in homogeneous mixture, as the distribution of the components is uniform, so we cannot judge a homogeneous mixture by just looking at it whereas in a heterogeneous mixture, due to the non-uniform composition of substance, one can easily identify it by just looking at the mixture.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




