
Which of the following is false about $ {H_2}{O_2}. $
(A) Act as both oxidizing and reducing agent
(B) Two OH bond lie in same plane
(C) Pale blue liquid
(D) Can be oxidized by ozone
Answer
535.8k+ views
Hint: $ {H_2}{O_2} $ also known as hydrogen peroxide has number of uses in chemistry like bleaching agent, antiseptic agent etc. and it contains a peroxide bond (which is Oxygen-Oxygen bond) and it does not have a planar structure because the molecule stabilizes in such a way that structure becomes non-planar.
Complete step by step solution:
$H_2O_2$ known as hydrogen peroxide is a colorless pale blue liquid in which peroxide bond is available (single oxygen-oxygen) it is formed when two molecules of hydrogen combine with two molecules of oxygen.
Some of the basic uses of $ {H_2}{O_2} $ are like using bleaching agents, oxidizer, and antiseptic.
If we discuss some of its chemical properties then, those are as follows: -
It acts as both oxidizing agent as well as reducing agent which can be clear by these following reactions: - Oxidation Reaction: - $ N{a_2}S{O_3}\; + {\text{ }}{H_2}{O_2}\; \to {\text{ }}N{a_2}S{O_4}\; + {\text{ }}{H_2}O $
Reduction Reaction: - $ Ba{O_2}\; + {\text{ }}{H_2}{O_2}\; \to {\text{ }}BaO{\text{ }} + {\text{ }}{H_2}O{\text{ }} + {\text{ }}{O_2} $
It can be easily oxidized by ozone as well. This is cleared by looking at the reaction of Hydrogen peroxide with ozone.
$ {O_{3\;}} + {\text{ }}{H_2}{O_{2\;}} \to {\text{ }}{H_2}O{\text{ }} + {\text{ }}2{O_2} $
Hence from this we can say ozone oxidizes hydrogen peroxide as well.
Hydrogen peroxide gets decomposed when exposed to sunlight, hence it is kept in dark bottles.
Structure of Hydrogen peroxide: -
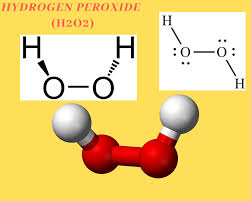
OH bond back of the plane
OH bond out of the plane
Hence by looking at the structure of Hydrogen peroxide, we can say the $2OH$ groups present are in different planes.
Therefore options A, C, D are correct.
Note:
In this question, specific properties of Hydrogen peroxide were asked and there are other few important chemicals like $ {H_2}S $ gas, Halogen compounds, Peracids and super-acids which are also important and their few basic properties are good to know which helps in answering similar such questions.
Complete step by step solution:
$H_2O_2$ known as hydrogen peroxide is a colorless pale blue liquid in which peroxide bond is available (single oxygen-oxygen) it is formed when two molecules of hydrogen combine with two molecules of oxygen.
Some of the basic uses of $ {H_2}{O_2} $ are like using bleaching agents, oxidizer, and antiseptic.
If we discuss some of its chemical properties then, those are as follows: -
It acts as both oxidizing agent as well as reducing agent which can be clear by these following reactions: - Oxidation Reaction: - $ N{a_2}S{O_3}\; + {\text{ }}{H_2}{O_2}\; \to {\text{ }}N{a_2}S{O_4}\; + {\text{ }}{H_2}O $
Reduction Reaction: - $ Ba{O_2}\; + {\text{ }}{H_2}{O_2}\; \to {\text{ }}BaO{\text{ }} + {\text{ }}{H_2}O{\text{ }} + {\text{ }}{O_2} $
It can be easily oxidized by ozone as well. This is cleared by looking at the reaction of Hydrogen peroxide with ozone.
$ {O_{3\;}} + {\text{ }}{H_2}{O_{2\;}} \to {\text{ }}{H_2}O{\text{ }} + {\text{ }}2{O_2} $
Hence from this we can say ozone oxidizes hydrogen peroxide as well.
Hydrogen peroxide gets decomposed when exposed to sunlight, hence it is kept in dark bottles.
Structure of Hydrogen peroxide: -

OH bond back of the plane
OH bond out of the plane
Hence by looking at the structure of Hydrogen peroxide, we can say the $2OH$ groups present are in different planes.
Therefore options A, C, D are correct.
Note:
In this question, specific properties of Hydrogen peroxide were asked and there are other few important chemicals like $ {H_2}S $ gas, Halogen compounds, Peracids and super-acids which are also important and their few basic properties are good to know which helps in answering similar such questions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




