
Which of the following is expected to be aromatic?

Answer
483.6k+ views
Hint: Aromatic compounds are those compounds which follow the Huckel rule of aromaticity. We will apply the Huckel rule of aromaticity to each of the given compounds and then find whether it is aromatic in nature or not. According to the Huckel rule, aromatic compounds must be planar and cyclic and they must contain $ \left( {4n + 2} \right){\text{ }}\pi $ electrons where n is a positive integer.
Complete answer:
We are given different organic compounds and we will identify the aromatic compound with the help of the Huckel rule of aromaticity. According to the Huckel rule, aromatic compounds are planar and cyclic in nature. These compounds must contain $ \left( {4n + 2} \right){\text{ }}\pi $ electrons where n is a positive integer and its value may start from zero to infinity. Also each double bond constitutes two pi electrons and negative also represent two pi electrons. Thus we will analyse each given compound as:
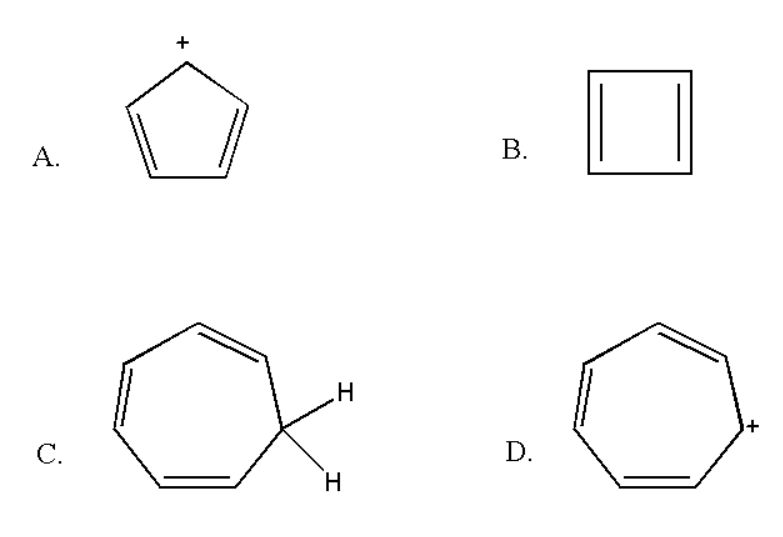
Here the number of double bonds is two and there is no negative charge. Thus it contain $ \left( {2 \times 2} \right){\text{ }} = {\text{ }}4 $ pi electrons which does not obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons. Thus it is not an aromatic compound.
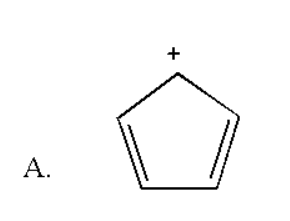
Here also the total number of pi bonds is two and thus it contains four pi electrons which do not obey Huckel rule of aromaticity.
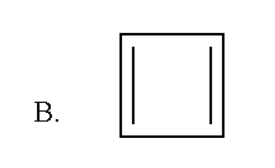
Here the total number of double bond is three and thus it contain $ \left( {2 \times 3} \right){\text{ }} = {\text{ 6}} $ pi electrons which obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons for $ n{\text{ }} = {\text{ }}1 $ . But it is not a planar molecule as hydrogen atoms will be out of the plane. Thus it does not obey Huckel's rule of aromaticity.
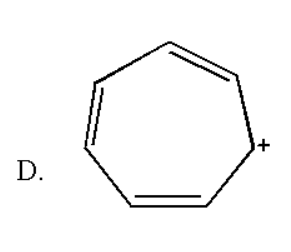
Here the total number of double bond is three and thus it contain $ \left( {2 \times 3} \right){\text{ }} = {\text{ 6}} $ pi electrons which obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons for $ n{\text{ }} = {\text{ }}1 $ . Also it is a planar molecule as there are no hydrogen atoms. Thus it obeys the Huckel rule of aromaticity.
Therefore we can say that the correct option is D.
Note:
It must be noted that for aromatic compounds all three conditions of Huckel rule must be satisfied which are planar, cyclic and $ \left( {4n + 2} \right){\text{ }}\pi $ electrons. If somehow it violates any one of the conditions then it is not an aromatic compound. Positive charges do not represent any electrons.
Complete answer:
We are given different organic compounds and we will identify the aromatic compound with the help of the Huckel rule of aromaticity. According to the Huckel rule, aromatic compounds are planar and cyclic in nature. These compounds must contain $ \left( {4n + 2} \right){\text{ }}\pi $ electrons where n is a positive integer and its value may start from zero to infinity. Also each double bond constitutes two pi electrons and negative also represent two pi electrons. Thus we will analyse each given compound as:

Here the number of double bonds is two and there is no negative charge. Thus it contain $ \left( {2 \times 2} \right){\text{ }} = {\text{ }}4 $ pi electrons which does not obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons. Thus it is not an aromatic compound.

Here also the total number of pi bonds is two and thus it contains four pi electrons which do not obey Huckel rule of aromaticity.

Here the total number of double bond is three and thus it contain $ \left( {2 \times 3} \right){\text{ }} = {\text{ 6}} $ pi electrons which obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons for $ n{\text{ }} = {\text{ }}1 $ . But it is not a planar molecule as hydrogen atoms will be out of the plane. Thus it does not obey Huckel's rule of aromaticity.

Here the total number of double bond is three and thus it contain $ \left( {2 \times 3} \right){\text{ }} = {\text{ 6}} $ pi electrons which obey rule of $ \left( {4n + 2} \right){\text{ }}\pi $ electrons for $ n{\text{ }} = {\text{ }}1 $ . Also it is a planar molecule as there are no hydrogen atoms. Thus it obeys the Huckel rule of aromaticity.
Therefore we can say that the correct option is D.
Note:
It must be noted that for aromatic compounds all three conditions of Huckel rule must be satisfied which are planar, cyclic and $ \left( {4n + 2} \right){\text{ }}\pi $ electrons. If somehow it violates any one of the conditions then it is not an aromatic compound. Positive charges do not represent any electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




