
Which of the following is capable of existing as a pair of enantiomers?
(A) $3 - {\text{Methylpentane}}$
(B) $3 - {\text{Methylhexane}}$
(C) $2 - {\text{Methylpentane}}$
(D) $2 - {\text{Methylpropane}}$
Answer
524.1k+ views
Hint: Isomers are divided into two groups called structural isomers and stereoisomers. Again stereoisomers are further classified into diastereomers and enantiomers. The distinguishing property of these two groups is the reflective symmetry of that molecule. Both diastereomers and enantiomers have different spatial configurations.
Complete step by step answer:
Diastereomers are not mere images of one another. Enantiomers are mere images of one another. Enantiomers are mirror images. The geometrical centre is called the chiral center. As enantiomers have similar spatial configuration, they have identical physical and chemical properties. They bend light in the opposite direction. The attachment of atoms is identical in enantiomers. Enantiomers have identical formulas.
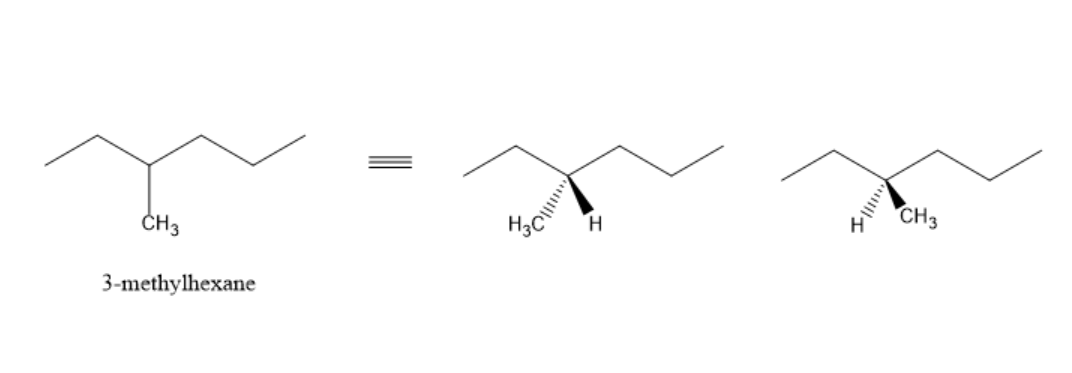
From the above diagram, we can observe that $3 - {\text{methylhexane}}$ exists as a pair of enantiomers. It is optically inactive due to molecular symmetry and chiral carbon.
Therefore, option B is correct.
Note: The compounds which are not superimposable with their mirror image are said to be chiral. Compounds which are superimposable with their mirror image are said to be achiral. An atom having four different substituents around it is called a stereocenter. Stereocenter is also called a stereogenic center. Properties like solubility and melting point are the same for enantiomers. If two enantiomers are present in equal proportions then the mixture is said to be a racemic mixture. Racemic mixture does not rotate polarized light because optical activity of each enantiomer is cancelled by another enantiomer.
Complete step by step answer:
Diastereomers are not mere images of one another. Enantiomers are mere images of one another. Enantiomers are mirror images. The geometrical centre is called the chiral center. As enantiomers have similar spatial configuration, they have identical physical and chemical properties. They bend light in the opposite direction. The attachment of atoms is identical in enantiomers. Enantiomers have identical formulas.

From the above diagram, we can observe that $3 - {\text{methylhexane}}$ exists as a pair of enantiomers. It is optically inactive due to molecular symmetry and chiral carbon.
Therefore, option B is correct.
Note: The compounds which are not superimposable with their mirror image are said to be chiral. Compounds which are superimposable with their mirror image are said to be achiral. An atom having four different substituents around it is called a stereocenter. Stereocenter is also called a stereogenic center. Properties like solubility and melting point are the same for enantiomers. If two enantiomers are present in equal proportions then the mixture is said to be a racemic mixture. Racemic mixture does not rotate polarized light because optical activity of each enantiomer is cancelled by another enantiomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




