
Which of the following is Aromatic Hydrocarbon?
A.${C_2}{H_2}$
B.${C_3}{H_8}$
C.${C_5}{H_{12}}$
D.${C_6}{H_6}$
Answer
577.8k+ views
Hint: Aromatic hydrocarbons are compounds which possess pleasant odor. Some contain benzene rings called benzenoids and some compounds do not contain a benzene ring known as non-benzenoids. Aromatic hydrocarbons have a general formula ${C_n}{H_{2n - 6y}}$ where y is no. of benzene rings and n should be equal to or greater than $6$.
Complete step by step answer:
Aromatic hydrocarbons are hydrocarbons which contain at least one special type of hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
First we will check each option.
${C_2}{H_2}$ is ethyne which is the simplest member of alkynes. Alkynes have a general formula${C_n}{H_{2n - 2}}$. It has at least one carbon-carbon triple bond.
${C_3}{H_8}$ is propane which is a member of alkane. Alkanes have a general formula${C_n}{H_{2n + 2}}$. It is a saturated hydrocarbon containing only carbon-carbon single bonds.
${C_5}{H_{12}}$is pentane which is also a member of alkane. Alkanes have a general formula${C_n}{H_{2n + 2}}$. It is a saturated hydrocarbon containing only carbon-carbon single bonds.
${C_6}{H_6}$ is benzene and it is aromatic hydrocarbon with general formula${C_n}{H_{2n - 6y}}$ where y is no. of benzene rings and n should be equal to or greater than$6$. If n=$6$ and y=$1$ then the formula becomes ${C_6}{H_{12 - 6}}$
On solving, we get-${C_6}{H_6}$
It is unsaturated and has hexagonal six carbon atoms with three double bonds in alternate positions. The structure is given as-
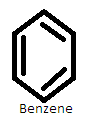
Also, it follows huckel’s rule according to which the aromatic compounds have delocalized $\left( {4n + 2} \right)$π electrons. So here n=$1$ and there are $6$π electrons. Thus benzene is aromatic.
The correct answer is option D.
Note:
Benzene is unsaturated and highly reactive in nature. There are following conditions the molecules to exhibit aromatic qualities-
-The molecule must have a planar structure where p-orbitals interact with each other.
-It should have cyclic structure and have a ring of p-orbitals which doesn’t have $s{p^3}$ hybridized atoms.
-There must be delocalized $\left( {4n + 2} \right)$π electrons in the system.
Complete step by step answer:
Aromatic hydrocarbons are hydrocarbons which contain at least one special type of hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
First we will check each option.
${C_2}{H_2}$ is ethyne which is the simplest member of alkynes. Alkynes have a general formula${C_n}{H_{2n - 2}}$. It has at least one carbon-carbon triple bond.
${C_3}{H_8}$ is propane which is a member of alkane. Alkanes have a general formula${C_n}{H_{2n + 2}}$. It is a saturated hydrocarbon containing only carbon-carbon single bonds.
${C_5}{H_{12}}$is pentane which is also a member of alkane. Alkanes have a general formula${C_n}{H_{2n + 2}}$. It is a saturated hydrocarbon containing only carbon-carbon single bonds.
${C_6}{H_6}$ is benzene and it is aromatic hydrocarbon with general formula${C_n}{H_{2n - 6y}}$ where y is no. of benzene rings and n should be equal to or greater than$6$. If n=$6$ and y=$1$ then the formula becomes ${C_6}{H_{12 - 6}}$
On solving, we get-${C_6}{H_6}$
It is unsaturated and has hexagonal six carbon atoms with three double bonds in alternate positions. The structure is given as-

Also, it follows huckel’s rule according to which the aromatic compounds have delocalized $\left( {4n + 2} \right)$π electrons. So here n=$1$ and there are $6$π electrons. Thus benzene is aromatic.
The correct answer is option D.
Note:
Benzene is unsaturated and highly reactive in nature. There are following conditions the molecules to exhibit aromatic qualities-
-The molecule must have a planar structure where p-orbitals interact with each other.
-It should have cyclic structure and have a ring of p-orbitals which doesn’t have $s{p^3}$ hybridized atoms.
-There must be delocalized $\left( {4n + 2} \right)$π electrons in the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




