
Which of the following is an anionic detergent?
A.) Sodium lauryl sulphate
B.) Cetyltrimethylammonium bromide
C.) Glyceryl oleate
D.) Sodium stearate
Answer
614.7k+ views
Hint: Anionic detergent are the detergents in which the lipophilic part of the molecule is an anion. In these types of detergent, anionic-active parts are most important. Anionic detergents are the sodium salts of the long-chain sulfonated alcohols or hydrocarbons.
Complete step-by-step answer:
We have to identify which among the following is an anionic detergent.
Let us discuss first the anionic detergents.
Anionic detergent is a type of synthetic detergent in which the lipophilic hydrocarbon group of the molecule is an anion.
A detergent molecule consists of a long hydrocarbon chain and a water-soluble negative ionic group.
These detergents are the sodium salts of the long-chain sulfonated alcohols or hydrocarbons.
Now, among the given options, the correct option is Sodium lauryl sulphate. It is an anionic detergent.
For better understanding refer to the structure of sodium lauryl sulphate shown below-
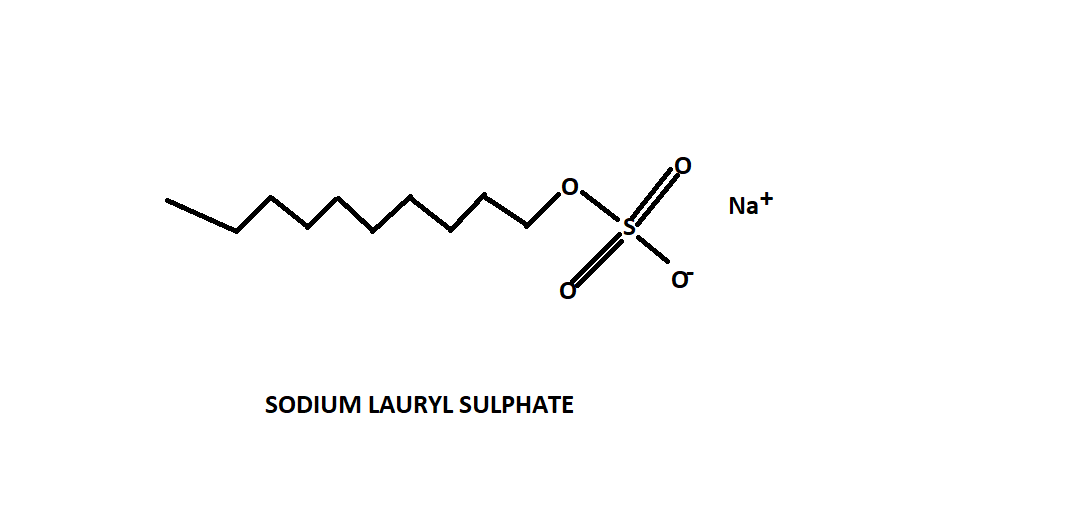 Sodium lauryl sulphate is an anionic detergent. It has anion at the soluble end of the chain. It is a sodium salt of the sulfonated long chain of lauryl alcohol.
Sodium lauryl sulphate is an anionic detergent. It has anion at the soluble end of the chain. It is a sodium salt of the sulfonated long chain of lauryl alcohol.
Hence, the correct option is option A.
Note – It is the most important point that while solving such types of questions, you should be well aware of the term anionic detergents and should not get confused with cationic detergents. There is a big difference among the two types. As mentioned in the solution, the anionic detergents contain anion at the soluble end of the long hydrocarbon chain. Whereas cationic detergents are, with a hydrophobic component of quaternary ammonium as the polar end. The ammonium centre is positively charged.
Complete step-by-step answer:
We have to identify which among the following is an anionic detergent.
Let us discuss first the anionic detergents.
Anionic detergent is a type of synthetic detergent in which the lipophilic hydrocarbon group of the molecule is an anion.
A detergent molecule consists of a long hydrocarbon chain and a water-soluble negative ionic group.
These detergents are the sodium salts of the long-chain sulfonated alcohols or hydrocarbons.
Now, among the given options, the correct option is Sodium lauryl sulphate. It is an anionic detergent.
For better understanding refer to the structure of sodium lauryl sulphate shown below-

Hence, the correct option is option A.
Note – It is the most important point that while solving such types of questions, you should be well aware of the term anionic detergents and should not get confused with cationic detergents. There is a big difference among the two types. As mentioned in the solution, the anionic detergents contain anion at the soluble end of the long hydrocarbon chain. Whereas cationic detergents are, with a hydrophobic component of quaternary ammonium as the polar end. The ammonium centre is positively charged.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Trending doubts
Who is known as the "Little Master" in Indian cricket history?

A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

Who Won 36 Oscar Awards? Record Holder Revealed

Why is there a time difference of about 5 hours between class 10 social science CBSE

Explain the Treaty of Vienna of 1815 class 10 social science CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




