
Which of the following is a substitution reaction?
A.
B.
C.
D.
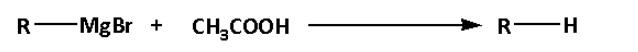



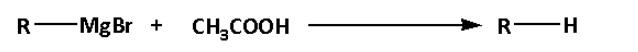
Answer
572.1k+ views
Hint:We know that a reaction in which the functional group of one compound is substituted by another functional group is known as a substitution reaction. In the substitution reaction, one atom or a group of atoms is replaced by another atom or a group of atoms.
Complete answer:
We are given four reactions.
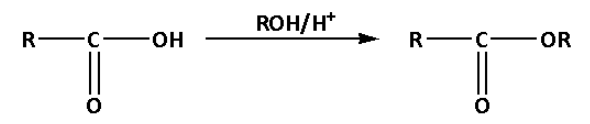
a. In reaction (A), the OH functional group is substituted by the (-OR) functional group.
In reaction (A), the carboxylic acid is converted to an ester by reaction with acid and alcohol. This reaction is known as Fischer esterification.
Thus, reaction (A) is a substitution reaction.
Thus, option (A) is correct.
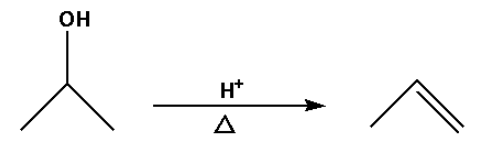
b. In reaction (B), an alcohol is converted to an alkene.
In reaction (B), the alcohol is converted to an alkene by heating the alcohol with a strong acid. The strong acids used are sulphuric acid, phosphoric acid. Water molecules are lost in the reaction.
Thus, reaction (B) is a dehydration reaction.
Thus, option (B) is not correct.
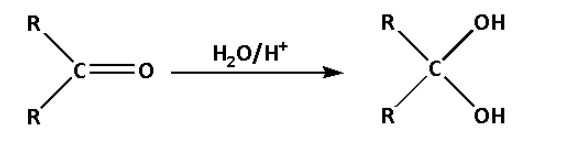
c. In reaction (C), ketone is converted to a diol. A diol is a chemical compound which contains two alcoholic functional groups.
In reaction (C), the ketone reacts with water in presence of an acid and forms a diol. Water molecules get added in the reaction.
Thus, reaction (C) is a hydration reaction.
Thus, option (C) is not correct.
d. In reaction (D), alkyl magnesium bromide reacts with acetic acid. The alkyl magnesium bromide is known as Grignard reagent.
In reaction (D), alkane is formed.
Thus, reaction (D) is not a substitution reaction.
Thus, option (D) is not correct.
Thus, the correct option is A.
Note:
In reaction (A), the hydroxyl (OH) group of the alcohol acts as a nucleophile and attacks the carbonyl carbon of the carboxylic acid. Thus, reaction (A) is a nucleophilic substitution reaction.
Complete answer:
We are given four reactions.
a. In reaction (A), the OH functional group is substituted by the (-OR) functional group.
In reaction (A), the carboxylic acid is converted to an ester by reaction with acid and alcohol. This reaction is known as Fischer esterification.
Thus, reaction (A) is a substitution reaction.
Thus, option (A) is correct.
b. In reaction (B), an alcohol is converted to an alkene.
In reaction (B), the alcohol is converted to an alkene by heating the alcohol with a strong acid. The strong acids used are sulphuric acid, phosphoric acid. Water molecules are lost in the reaction.
Thus, reaction (B) is a dehydration reaction.
Thus, option (B) is not correct.
c. In reaction (C), ketone is converted to a diol. A diol is a chemical compound which contains two alcoholic functional groups.
In reaction (C), the ketone reacts with water in presence of an acid and forms a diol. Water molecules get added in the reaction.
Thus, reaction (C) is a hydration reaction.
Thus, option (C) is not correct.
d. In reaction (D), alkyl magnesium bromide reacts with acetic acid. The alkyl magnesium bromide is known as Grignard reagent.
In reaction (D), alkane is formed.
Thus, reaction (D) is not a substitution reaction.
Thus, option (D) is not correct.
Thus, the correct option is A.
Note:
In reaction (A), the hydroxyl (OH) group of the alcohol acts as a nucleophile and attacks the carbonyl carbon of the carboxylic acid. Thus, reaction (A) is a nucleophilic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




