
Which of the following is a secondary radical?
a) $C{{H}_{2}}=CH-$
b) ${{(C{{H}_{3}})}_{3}}C-$
c) ${{C}_{6}}{{H}_{5}}-$
d) $C{{H}_{3}}-{{(C{{H}_{2}})}_{2}}-C{{H}_{2}}-$
Answer
617.4k+ views
Hint: Remember that the degree of a radical is determined by the number of carbon atoms directly attached to the carbon radical.
For example, an ethyl radical ($C{{H}_{3}}-C{{H}_{2}}-$) is of the first degree and is referred to as a primary radical since the radical carbon atom is only attached to one other carbon atom.
Therefore, the correct option’s radical atom must be connected to two other carbon atoms, thereby making it a secondary radical.
Complete step-by-step solution:
Let us establish what a radical is before we use the above hint to help solve the question.
Radical, also called Free Radical, in chemistry, a molecule that contains at least one unpaired electron.
Most molecules contain an even number of electrons, and the covalent chemical bonds holding the atoms together within a molecule normally consist of pairs of electrons jointly shared by the atoms linked by the bond.
Most radicals may be considered to have arisen by cleavage of normal electron-pair bonds, every cleavage having produced two separate entities, each of which contains a single, unpaired electron from the broken bond (in addition to all the rest of the normal, paired electrons of the atoms).
Now, that we know what a radical really is, let's try to find the answer by finding the degree of each individual option.
$C{{H}_{2}}=CH-$
In this particular radical, the carbon radical is connected only to one other carbon atom, therefore making it a primary radical.
Additional information:
This is the radical of ethene which is a crucial part of the polymerisation of ethene which leads to the formation of polyethene)
${{(C{{H}_{3}})}_{3}}C-$
In this particular radical, the carbon radical is connected to three other carbon atoms, therefore making it a tertiary radical.
${{C}_{6}}{{H}_{5}}-$
To understand the degree of this radical, we need to draw its figure first.
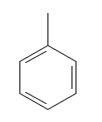
Now we observe that, in this radical, the carbon radical is attached to two other Carbon atoms regardless of which atom in this ring acts as the radical. Therefore, this is a secondary radical. (Additional information: This is the benzyl radical which is used in the production of many aromatic compounds.)
$C{{H}_{3}}-{{(C{{H}_{2}})}_{2}}-C{{H}_{2}}-$
In this particular radical, the carbon radical is connected only to one other carbon atom, therefore making it a primary radical.
Therefore, through this analysis, we can conclude that the answer is
c) ${{C}_{6}}{{H}_{5}}-$
NOTE: To help solve this question correctly, it is advisable to draw all of the chemical structures of these radicals before giving an answer for more effective visualisation.
For example, an ethyl radical ($C{{H}_{3}}-C{{H}_{2}}-$) is of the first degree and is referred to as a primary radical since the radical carbon atom is only attached to one other carbon atom.
Therefore, the correct option’s radical atom must be connected to two other carbon atoms, thereby making it a secondary radical.
Complete step-by-step solution:
Let us establish what a radical is before we use the above hint to help solve the question.
Radical, also called Free Radical, in chemistry, a molecule that contains at least one unpaired electron.
Most molecules contain an even number of electrons, and the covalent chemical bonds holding the atoms together within a molecule normally consist of pairs of electrons jointly shared by the atoms linked by the bond.
Most radicals may be considered to have arisen by cleavage of normal electron-pair bonds, every cleavage having produced two separate entities, each of which contains a single, unpaired electron from the broken bond (in addition to all the rest of the normal, paired electrons of the atoms).
Now, that we know what a radical really is, let's try to find the answer by finding the degree of each individual option.
$C{{H}_{2}}=CH-$
In this particular radical, the carbon radical is connected only to one other carbon atom, therefore making it a primary radical.
Additional information:
This is the radical of ethene which is a crucial part of the polymerisation of ethene which leads to the formation of polyethene)
${{(C{{H}_{3}})}_{3}}C-$
In this particular radical, the carbon radical is connected to three other carbon atoms, therefore making it a tertiary radical.
${{C}_{6}}{{H}_{5}}-$
To understand the degree of this radical, we need to draw its figure first.

Now we observe that, in this radical, the carbon radical is attached to two other Carbon atoms regardless of which atom in this ring acts as the radical. Therefore, this is a secondary radical. (Additional information: This is the benzyl radical which is used in the production of many aromatic compounds.)
$C{{H}_{3}}-{{(C{{H}_{2}})}_{2}}-C{{H}_{2}}-$
In this particular radical, the carbon radical is connected only to one other carbon atom, therefore making it a primary radical.
Therefore, through this analysis, we can conclude that the answer is
c) ${{C}_{6}}{{H}_{5}}-$
NOTE: To help solve this question correctly, it is advisable to draw all of the chemical structures of these radicals before giving an answer for more effective visualisation.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

Write the formula to find the shortest distance between class 12 maths CBSE




